Abstract
The purpose of our study was to analyze the incidence of incidental thyroid cancers which were detected by simultaneous sonographic examination of breast and thyroid glands. Between January 2001 and March 2004, 518 patients were diagnosed with breast cancer after modified radical mastectomy (n = 369) or breast conserving surgery (n = 149). We screened thyroid glands when we examined breast for diagnosis and follow-up after surgery. If we found the sonographic finding of suspicious for malignancy in thyroid, we immediately performed ultrasound-guided fine needle aspiration biopsy (FNAB). Forty-two cases showed suspicious sonographic findings and of those, 18 cases (42.9%) were determined to have suspicious malignant cytology by ultrasound guided FNAB. Among 518 breast cancers, total 13 cases (2.5%) were diagnosed with papillary carcinoma after thyroidectomy. The mean longest diameter of the thyroid masses was 9.9mm (range 1 - 30mm). Six cases (6/13, 46.2%) were diagnosed as simultaneous breast and thyroid cancers, and the rest of the thyroid cancers were detected after 6 to 33 months (mean 16.5 months) after surgery. In conclusion, the patients with breast cancer had a high incidence (2.5%) of thyroid cancer. Sonographic screening is useful for the early detection of thyroid cancer.
Breast cancer is the most frequent malignant tumor in Korean women since 2001.1 With advances in therapeutic methods and screening, many breast cancer survivors have undergone follow-up studies, which have revealed secondary malignancies such as gynecologic malignancies, thyroid cancer and lymphoma.2-4
Previous studies have reported a higher incidence of thyroid cancer in women with breast cancer, and researchers have supposed that genetic, hormonal factors or radiation effects could be the etiologic factors.4-7
The incidence of thyroid cancer is currently increasing in many countries.8-10 Examination of a patient by palpation has been the traditional screening method for thyroid cancer,11,12 but the introduction of high-resolution sonography has made sonographic screening possible. A few studies have reported on the screening for thyroid cancer in high-risk groups, but there are few reports about the sonographic screening in breast cancer patients.13-16
Therefore, we analyzed the incidence of thyroid cancer detected by simultaneous sonographic examination of breast and thyroid glands.
This study was approved by institutional review board of Yongdong Severance Hospital and all patients gave written form of informed consent for the thyroid sonography.
Between January 2001 and March 2004, 518 women (aged 22-78 years, mean age 46.5 ± 7.2 years) were diagnosed as breast cancer proven by pathology after either modified radical mastectomy (n = 369) or breast conserving surgery (n = 149). Of the 518 breast cancers, 207 patients (40.0%) received subsequent radiotherapy at the breast only (n = 113) or the breast with supraclavicular area (n = 94). The mean dose of radiation was 5,040cGy.
All breast cancer patients underwent follow-up breast sonography after the mastectomy every 6 months for the first 2 or 3 years and annually thereafter (range 4 to 39 months, mean 18.3 months). The pathology of breast cancer varied and is listed in Table 1.
We examined thyroid glands of the breast cancer patients during an initial and follow-up breast sonography with 10 - 12MHz probes of HDI 5000 or 3000 scanners (Advanced Technology Laboratories, Bothell, WA, USA). Three radiologists, with at least six years experience of breast and thyroid sonography performed the scans. When the sonographic findings of the thyroid showed one or more malignant characteristics,15 such as an ill-defined margin, hypoechogenicity, microcalcification or taller-than-wider shape, we immediately performed ultrasound (US)-guided fine needle aspiration biopsy (FNAB). We included complex cysts with same criteria for solid portion within the nodules.
Among 518 cases, 42 patients (8.1%) had at least one suspicious sonographic finding including ill-defined hypoechoic nodules (n = 28), taller-than-wider hypoechoic nodules (n = 16), microcalcifications (n = 14) and complex cysts with suspicious findings (n = 11). Among them, eleven patients (26.2%) had multiple nodules.
Eighteen cases (18/42, 42.9%) had suspicious or malignant cytology by ultrasound guided FNAB, which included the reports of suggestive of malignancy (n = 11) and suspicious for malignancy (n = 7). Among 18 cases, 5 cases were diagnosed as adenomatous hyperplasia (n = 3) and follicular adenoma (n = 2) after surgery. Finally, a total of 13 cases (13/518, 2.5%) were diagnosed as papillary carcinoma after a thyroidectomy including four patients with bilateral thyroid cancers (Table 2). The long diameter of the thyroid masses ranged 1 - 30mm (mean 9.9mm), and microcarcinoma (cancer less than 1cm) was the diagnosis in 8 cases (61.5%).
Three cases (23.1%) had extracapsular invasion. Two cases (15.4%) had central compartment lymph node metastasis, and of these, one case had lateral neck node metastasis. No thyroid cancer patient showed distant metastasis.
Six cases of 13 thyroid cancer patients (46.2%) were diagnosed as simultaneous breast and thyroid cancers (Fig. 1), and the rest of the thyroid cancers were detected 7 to 33 months (mean 16.5 months) after mastectomy (Fig. 2). Among seven patients, thyroid cancer followed breast cancer. In four patients, thyroid cancer was detected at the 4 months (n = 2) and 9 months (n = 2) follow-up after radiotherapy.
Several investigators have reported an increase in thyroid cancer in their countries.8-10 In Korea, thyroid cancer is the most rapidly increasing cancer in women, according to the 2001 Annual Report of the Korea Central Cancer Registry in 2004.1 There is a distinct difference between the prevalence of occult cancer and clinical cancer. The prevalence of occult thyroid cancer is higher (5 - 35%) in autopsy series than clinically apparent cancer (1.4 - 4.5/100,000).6,10,17
The prognosis of thyroid cancer varies from indolent papillary carcinomas to fatal anaplastic carcinomas. Papillary and follicular carcinomas make up about 90% of all thyroid cancers.10,18 Most thyroid cancers are differentiated carcinomas and the investigated prognosis is excellent with a cancer-specific mortality of less than 1 - 2%.18,19 By reason of the favorable prognosis, treatment of small thyroid cancer remains controversial.20,21 However, it should be considered that even occult carcinoma could be associated with distant metastasis.19,21
In this study, mean tumor size was 9.9mm and microcarcinoma made up 61.5% of the total number of cases, and all thyroid cancers were well-differentiated papillary carcinomas. Miki et al.11 reported that tumors in the screening group were significantly smaller than in the outpatient group, and the incidence of regional lymph node metastasis in the screening group was significantly lower in the outpatient group. According to several screening studies, screening-detected thyroid cancers were well-differentiated cancers and an excellent prognosis was expected.11,12
The standard screening method of thyroid cancer was physical examination. However, sonographic screening using high frequency probe has been recently studied.14,15 The incidence of thyroid cancer in the sonographic screening was 0.76%, and the incidence was significantly higher in breast cancer group (1.9%) than that of non-breast cancer group (0.6%).15 Previous studies have reported an increased occurrence of thyroid cancer in breast cancer patients and an increased occurrence of breast cancer in thyroid cancer patients. The relationship has not been clearly determined, but there are many studies about the possible causes.4-7 Genetic and hormonal factors may play a role in this concurrence. Thyroid cancer is 2 - 4 times more frequent in females than in males. The incidence is approximately the same for males and females before puberty and after menopause, which suggests an effect of estrogen. Exposure to ionized radiation is a known risk factor for both thyroid cancer and breast cancer, but there are controversies about the adverse effect of radiation therapy used for the primary cancer.22 In this study, considerable thyroid cancers (46.1%) already existed at the time of breast cancer diagnosis. There was no significant relationship between thyroid cancer and radiation therapy for breast cancer. But short-term follow-up to identify the influence of radiotherapy was a limitation in our study, considering a previous report, which stated that radiation-induced thyroid cancer usually increases with time-dependency.23
Weiss et al. supposed that an increase in secondary malignancy is due to an increase in medical surveillance of patients after the diagnosis of primary cancer.24
We could find very few reports about the relationship of pathologic type of breast cancer to thyroid cancer. In our study, thyroid cancers were detected in invasive ductal carcinoma and DCIS (ductal carcinoma in situ) cases. But the high proportion of these pathologies in breast cancer may cause this result and more studies regarding this relationship should be pursued.
Several US findings have been studied as predictors for thyroid cancer, but there is overlap in their appearance.16,25,26 Therefore, US-guided FNAB is widely used diagnostic tool because of the safety, inexpensiveness and high sensitivity.16,26-28 Many sonographic criteria have been used for recommending FNAB,16,25,26 and the common criteria were microcalcification, irregular margin, and hypoechogenicity. But they were focused on solid nodule and papillary carcinoma. More accurate guideline should be considered for diagnosis of non-papillary carcinoma or cystic nodules.
Despite of high accuracy of FNAB, cytology-histology discrepancy can be existed, especially for adenomatous hyperplasia or follicular neoplasm.27,29,30 In this study, five cases of false positive cytology were adenomatous hyperplasia (n = 3) and follicular adenoma (n = 2). Recent studies recommended repeat FNAB for the indeterminate or suspicious cytology.31
In conclusion, patients with breast cancer had a high incidence (2.5%) of thyroid cancer. Sonography can be a good modality for the early detection of thyroid cancer.
Figures and Tables
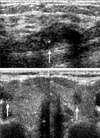 | Fig. 138-year-old woman with breast and thyroid cancer simultaneously. (A) Breast sonography showed irregular hypoechoic mass with microcalcifications (arrow). (B) Simultaneous thyroid sonography showed about 5mm-sized hypoechoic nodules on both sides (arrows). Infiltrating ductal carcinoma of the breast and papillary carcinoma of both thyroid glands were confirmed. |
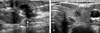 | Fig. 251-year-old woman with breast cancer; thyroid cancer was detected 12 months after mastectomy. (A) Preoperative breast sonography showed an irregular hypoechoic mass (arrow) at left subareolar area, proving infiltrating ductal carcinoma. Initial thyroid sonography was negative. (B) 12-month follow-up sonography showed an approximately 5mm-sized taller-than-wider hypoechoic nodule at the right thyroid gland, proving papillary carcinoma. |
References
1. Shin HR, Won YJ, Jung KW, Park JG. 2001 Annual Report of the Korea Central Cancer Registry: based on registered data from 134 hospitals. Cancer Res Treat. 2004. 36:19–30.

2. Tanaka H, Tsukuma H, Koyama H, Kinoshita Y, Kinoshita N, Oshima A. Second primary cancers following breast cancer in the Japanese female population. Jpn J Cancer Res. 2001. 92:1–8.

3. Matesich SM, Shapiro CL. Second cancers after breast cancer treatment. Semin Oncol. 2003. 30:740–748.

4. Rubino C, de Vathaire F, Shamsaldin A, Labbe M, Le MG. Radiation dose, chemotherapy, hormonal treatment and risk of second cancer after breast cancer treatment. Br J Cancer. 2003. 89:840–846.

5. Li CI, Rossing MA, Voigt LF, Daling JR. Multiple primary breast and thyroid cancers: role of age at diagnosis and cancer treatments (United States). Cancer Causes Control. 2000. 11:805–811.
6. Colonna M, Grosclaude P, Remontet L, Schvartz C, Mace-Lesech J, Velten M, et al. Incidence of thyroid cancer in adults recorded by French cancer registries (1978-1997). Eur J Cancer. 2002. 38:1762–1768.

7. Chen AY, Levy L, Goepfert H, Brown BW, Spitz MR, Vassilopoulou-Sellin R. The development of breast carcinoma in women with thyroid carcinoma. Cancer. 2001. 92:225–231.

8. Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J. Cancer incidence in five continents. 1997. vol. VII. Lyon, France: International Agency for Research on Cancer, Scientific Publication;no. 143.
9. Liu S, Semenciw R, Ugnat AM, Mao Y. Increasing thyroid cancer incidence in Canada, 1970-1996: time trends and age-period-cohort effects. Br J Cancer. 2001. 85:1335–1339.

10. Reynolds RM, Weir J, Stockton DL, Brewster DH, Sandeep TC, Strachan MW. Changing trends in incidence and mortality of thyroid cancer in Scotland. Clin Endocrinol (Oxf). 2005. 62:156–162.

11. Miki H, Inoue H, Komaki K, Uyama T, Morimoto T, Monden Y. Value of mass screening for thyroid cancer. World J Surg. 1998. 22:99–102.

12. Eden K, Mahon S, Helfand M. Screening high-risk populations for thyroid cancer. Med Pediatr Oncol. 2001. 36:583–591.

13. Ezzat S, Sarti DA, Cain DR, Braunstein GD. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994. 154:1838–1840.

14. Lee HK, Hur MH, Ahn SM. Diagnosis of occult thyroid carcinoma by ultrasonography. Yonsei Med J. 2003. 44:1040–1044.

15. Park JS, Oh KK, Kim EK, Chang HS, Hong SW. Sonographic screening for thyroid cancer in female undergoing breast sonography. Am J Roentgenol. 2006. 186:1025–1028.

16. Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. Am J Roentgenol. 2002. 178:687–691.

17. Nagataki S, Nystrom E. Epidemiology and primary prevention of thyroid cancer. Thyroid. 2002. 12:889–896.

19. Hay ID, Grant CS, van Heerden JA, Goellner JR, Ebersold JR, Bergstralh EJ. Papillary thyroid microcarcinoma: a study of 535 cases observed in a 50-year period. Surgery. 1992. 112:1139–1147.
20. Ringel MD, Ladenson PW. Controversies in the follow-up and management of well-differentiated thyroid cancer. Endocr Relat Cancer. 2004. 11:97–116.

21. Noguchi S, Yamashita H, Murakami N, Nakayama I, Toda M, Kawamoto H. Small carcinomas of the thyroid. A long-term follow-up of 867 patients. Arch Surg. 1996. 131:187–191.
22. Huang J, Walker R, Groome PG, Shelly W, Mackillop WJ. Risk of thyroid carcinoma in a female population after radiotherapy for breast carcinoma. Cancer. 2001. 92:1411–1418.

23. Ron E, Lubin JH, Shore RE, Mabuchi K, Modan B, Pottern LM, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995. 141:259–277.

24. Weiss NS, Rossing MA. Healthy screened bias in epidemiologic studies of cancer incidence. Epidemiology. 1996. 7:319–322.
25. Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Management of thyroid nodules detected at US: Society of radiologists in ultrasound consensus conference statement. Radiology. 2005. 237:794–800.

26. Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002. 5:1941–1946.

27. Gharib H, Goellner JR. Fine-Needle aspiration biopsy of the thyroid: an appraisal. Ann Intern Med. 1993. 118:282–289.

28. Kim SJ, Kim EK, Park CS, Chung WY, Oh KK, Yoo HS. Ultrasound-guided fine-needle aspiration biopsy in nonpalpable thyroid nodules: is it useful in infracentimetric nodules? Yonsei Med J. 2003. 44:635–640.

29. Schlinkert RT, van Heerden JA, Goellner JR, Gharib H, Smith SL, Rosales RF, et al. Factors that predict malignant thyroid lesions when fine-needle aspiration is "suspicious for follicular neoplasm". Mayo Clin Proc. 1997. 72:913–916.

30. Settakorn J, Chaiwun B, Thamprasert K, Wisedmongkol W, Rangdaeng S. Fine needle aspiration of the thyroid gland. J Med Assoc Thai. 2001. 84:1401–1406.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download