Abstract
Avian influenza has emerged as one of the primary public health concern of the 21st century. Influenza strain H5N1 is capable of incidentally infecting humans and other mammals. Since their reemergence in 2003, highly pathogenic avian influenza A (H5N1) viruses have been transmitted from poultry to humans (by direct or indirect contact with infected birds) in several provinces of Mainland China, which has resulted in 22 cases of human infection and has created repercussions for the Chinese economy. People have been concerned whether a new pandemic will occur in the future. The eradication of pathogenic avian influenza viruses appears to be the most effective way to prevent an influenza pandemic. This paper will examine the features of H5N1, including incidence, infection, immunity, clinical management, prevention and control, and therapy in Mainland China.
Over the last 20 years, new and reemerging pathogens (e.g., West Nile Virus, anthrax) have appeared at alarming rates, garnering considerable attention from the scientific and public health communities, as well as from the media. The Chinese, who recently dealt with the terror caused by Severe Acute Respiratory Syndrome (SARS), now face the threat posed by avian influenza. Avian influenza is commonly known as "bird flu" and has caused enormous economic loss for China.1 This review will discuss the features of highly pathogenic avian influenza viruses (H5N1), incidence, infection, immunity, clinical management, prevention, and control in Mainland China.
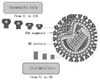
Avian influenza virus is a single-stranded RNA virus of the Orthomyxoviridae family. Influenza viruses are classified into groups A, B and C according to differences in the nucleocapsid and matrix protein.2 The type A viruses were divided into haemagglutinin (H) and neuraminidase (N) subtypes based on the surface glycoproteins.3,4 Up until to now, 16 H and 9 N subtypes have been reported (Fig. 1).5,6 All influenza subtypes can be found in waterfowl, but only the H 1-3 and N 1-2 subtypes were known to infect humans; in particular, the H5N1 subtype is highly pathogenic and endemic in Asian nations including Mainland China. In 1997, the H5N1 virus was first isolated from a three-year-old boy in Hong Kong,7 and new genotypes of H5N1 virus have continually emerged in Mainland China.8 H5N1 virus infection has three features. First, the area where avian influenza cases occurred did not have animal plague and the cases of H5N1 showed dissemination in Mainland China.9 Secondly, the outbreak was mainly distributed in Central, East China and South China; in South China the outbreak was more serious. Third, the virus pathogenicity was strong. The H5N1 subtypes not only caused the death of fowl, ducks, goose and turkey, but also caused human morbidity(Fig. 2).10-12
The confirmed cases of avian influenza A (H5N1) in humans since 2003 have been listed in Table 1.13-15 From this table, it was found that the avian influenza plague had been reported in several cities of Mainland China in 2003 and 2004. However, no cases had been diagnosed in humans.16 At the same time it was also found that most cases appeared in females, which may be associated with genotype, although to determine the true reason required further research, and the frequencies of H5N1 infection among humans have not been reported. Human influenza is transmitted by way of the inhalation of infectious droplets and droplet nuclei by direct or indirect contact.17 There were three primary routes for the transmission of avian influenza, the first of which was by contact with the domestic fowl market; the second by bird migrations; and the third was by the transmission from fowl to mammals. Additionally, the infected birds, which were captured, led to poultry infection, although this type of transmission was merely a possibility. Avian influenza usually involves horizontal transmission, while the vertical transmission of the virus has not been discovered. However, the H5N2 subtype has been isolated from chicken eggs, indicating a possibility for vertical transmission.18 For human influenza A (H5N1), the main transmission routes were from birds to humans and from the environment to humans. Human-to-human transmission of influenza A (H5N1) has been suggested in several household clusters, and there was also one case of possible child-to-mother transmission. Intimate contact without the use of protective measures was implicated, and no case of human-to-human transmission by small-particle aerosols was identified.19 In 1997, human-to-human transmission apparently did not occur through social contact, and serologic studies of exposed health care workers showed that the transmission was inefficient. Recently, some researchers have suggested that the animal virus strains may be adapting to humans by accumulating nonsynonymous (amino acid-changing) substitutions in key proteins (e.g. surface glycoprotein).20 However, epidemiologic and virologic studies are needed to confirm whether avian influenza viruses have acquired the capacity for human infection by adaptive evolution. To date, the risk of nosocomial transmission to health care workers has been low.
The early stage of an infection is often viewed as a race between the virus and the host's immune system. The first layer of immunity against virus invasion is the integrity of the body surface. Once breached, early 'non-specific' or innate immunity defenses, such as interferons, macrophages and natural killer (NK) cells, become active.21 Type 1 interferons have a primary protective function in the early stages of influenza virus infection.22 Interferons can induce the expression of 'Mx' genes, which can inhibit primary transcription of the viral PB2 polymerase.23,24 To combat this immune response, viruses employ an 'NS' gene that encodes a type 1 interferon antagonist that promotes virus growth.25 Although interferon-γ (IFN-γ) has an obvious function against other viruses, there are no findings to date to support that this cytokine can control influenza viruses.26 Similarly, no involvement of IFN-γ-producing natural killer cell has been identified. However, some evidence indicates that α-galactosylceramide-stimulated natural killer T cells may be of some benefit.27
Evidence showed that the H5N1 virus mainly attaches to type II pneumocytes, alveolar macro-phages and nonciliated bronchiolar cells in the human lower respiratory tract (LRT).28 Some researchers have also found that the number of alveolar macrophages increases during an early influx of neutrophils into the infected lung, which may imply that alveolar macrophages may be protective during the initial stage of infection.29
An absence of T cells renders the host highly susceptible to viral attack. Although our knowledge of avian influenza cellular immunology has expanded rapidly in the last decade, very little was known about the importance of cellular immunity against the avian influenza virus.30 The influenza antigens may travel to the regional cervical and mediastinal lymph nodes or to the spleen by dendritic cells (DCs), which exit at the respiratory tract.31 The amount of antigen-specific response that is generated by direct exposure to migrated DCs or by cross-presentation on resident lymphoid tissue DCs is unclear.32 Studies have found that only the CD8α+ DCs can stimulate influenza peptide-specific T cells or hybridoma cell lines.33 Analysis of the CD8+ T cell response has indicated that all antigen-presenting cells (APCs) could eliminate virus infection within 10-14 days.34 However, experiments using T cell receptor (TCR)-transgenic CD4+ T cells have suggested that APCs are capable of stimulating influenza-specific CD4+ T cells with lower efficiency, and this process can still be occurring for three weeks after initial viral exposure.35,36 No evidence indicates that either viral RNA or antigen can induce immune responses for longer periods.
The humoral immune response in naturally infected poultry includes systemic and mucosal antibody production.37 Antibodies provide a major barrier to virus spread between cells and tissues, especially in restricting virus spread in the blood stream. Antibodies may be generated against any viral protein in the infected cell. Antibodies against glycoproteins expressed on the virion envelop or on the infected cell membrane are of importance in controlling infection.21,38 Influenza viruses produce ten viral proteins that can be divided into three main categories: surface proteins, internal proteins, and nonstructural proteins.39,40 The surface proteins, which are the only antigens capable of inducing neutralizing antibodies, include three types of proteins: Haemagglutinin (HA), Neuraminidase (NA), and Matrix 2 (M2) proteins.41,42
The HA protein has two main functions: one is that of the virus receptor binding site; and the other is used to fuse the viral RNA that is released into the host cell.43 Regarding HA, at least five antigenic epitopes have been discovered for human influenza viruses, and each epitope was capable of inducing neutralizing antibodies. Antibodies against the HA protein were the main determinant for protection in the host against disease, and vaccine for influenza in poultry is primarily based on the HA subtype. The importance of the HA protein requires further investigation.44 The NA protein is an enzymatically active protein that is important in allowing the virus to be released from the cell surface.45 The NA protein also induces neutralizing antibodies in chickens. Antibodies to the NA protein are of less importance than those to the HA protein.46
The M2 protein (M2e) is an integral membrane protein that functions as an ion channel for the virus particle. The antibody to the M2e protein in mice does not provide complete protection, although it does reduce the amount of virus that is shed and provides some protection from the disease. However, the antibody to the M2e protein is well conserved for all influenza type A viruses and potentially could provide protection for all HA and NA subtypes.47 Recent studies found that human influenza vaccines based on the extracellular domain of influenza M2e induced broad-spectrum protective immunity in various antigen constructs.48 The mucosal immune response also probably has a role in protection from H5N1 infection since the initial exposure to the virus is through a mucosal surface. However, little direct work has been done regarding the mucosal immune response in chickens and turkeys.49 Although both antibodies and T cells contribute to the response against influenza virus infections, the viral antigens are less recognizable by the host's acquired immunity because of "antigenic drift".50 As a result of antigenic drift, avian influenza viruses can escape host immune surveillance. An even more radical method of immune evasion occurs when the segmented structure of influenza A genome allows swapping of major gene segments when two different influenza A virus strains infect the same cell.51 At the same time, influenza A virus NS1 protein can not only inhibit innate immunity by preventing type 1 IFN release, but also can inhibit adaptive immunity by attenuating human DC maturation and decreasing the number of DCs available to induce T-cell responses.52,53
The current danger to people from avian influenza has been recognized. The World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) have adopted measures to prevent the emergence of avian influenza in Asia and control its wider transmission.54 The CDC's response has been focused on enhancing surveillance and laboratory testing for human avian influenza (Table 2).55
The Chinese government also exerts great importance on the prevention and control of avian influenza. The Chinese Center for Disease Control and Prevention has published interim guidelines to limit the possibility of human infections during outbreaks of avian influenza in domestic birds and poultry in Mainland China.56-59 In addition, the Chinese government has taken a series of concrete measures in this regard:
Influenza A (H5N1) virus is susceptible to Oseltamivir (Tamiflu) and Zanamivir (Relenza)70 but is resistant to Amantadine and Rimantadine.71,72 Treatment should be started within 48 hours of onset of fever, without waiting for laboratory confirmation.73,74 Mild cases are treated. A higher dose of 150mg twice daily and treatment for 7 to 10 days is required for the treatment of severe infections. Salicylate administration should be avoided in children younger than 18 years to prevent the possibility of Reye's syndrome. The effectiveness of Zanamivir in reducing the severity and duration of illness and in preventing complications has been proven in children from 5 to 12 years old.75
The Chinese government learned a lesson during the SARS outbreak. During the threat of avian influenza pandemic, the Chinese Government has taken a series of open, preventive and surveillance measures to control avian influenza transmission.76 Infected fowl have been the primary source of H5N1 infections in humans in Mainland China. At present, transmission between humans is limited, although long-term monitoring is required to identify viral adaptation to human hosts. With increasing poultry production worldwide, it is not a question whether a new pandemic strain will emerge but rather when it will emerge. This matter requires the concerted efforts of poultry producers to improve biosecurity. Governments also will need to intensify their efforts to educate their citizens concerning the risks of avian influenza.
ACKNOWLEDGEMENT
We thank Miss Cao Hui for correcting this manuscript and also thank Zhu Haitao for collecting materials.
References
1. Zhang M. Chinese economic loss USD 87,000,000,000 because for avian influenza Oriental Morning Post. 2005. Nov 22. Available from http://finance.sina.com.cn/g/20051122/07132136780.shtml. Chinese.
2. Lahariya C, Sharma AK, Pradhan SK. Avian flu and possible human pandemic. Indian Pediatr. 2006. 43:317–325.
3. Horimoto T, Kawaoka Y. Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol. 2005. 3:591–600.

4. Xu XY, Wang QH. Biology and epidemiology of avian influenza. Zhonghua Yi Xue Za Zhi. 2004. 84:353–354.
5. Liu JP. Avian influenza-a pandemic waiting to happen? J Microbiol Immunol Infect. 2006. 39:4–10.
6. Beijing Centers for Diseases Control and Prevention & Centers Preventive Medical Research. Schematic diagram of avian influenza subtypes virus. Xinhua News Agency. 2006. Feb 21. Aviable from http://news.sina.com.cn/c/p/2006-02-21/20219164152.shtml. Chinese.
7. Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998. 279:393–396.

8. Claas EC, Osterhaus AD, van Beek R, De Jong JC, Rimmelzwaan GF, Senne DA, et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998. 351:472–477.

9. Chen H, Deng G, Li Z, Tian G, Li Y, Jiao P, et al. The evolution of H5N1 influenza viruses in ducks in southern China. Proc Natl Acad Sci U S A. 2004. 101:10452–10457.

10. Xing L. The Chinese avian influenza case presentd disperse and Ministry of Public Health asked to make careful obviate chinanews.com. 2006. April 28. Available from http://www.chinanews.com.cn//news/2006/2006-04-28/8/724249.shtml.Chinese.
11. The news office of State council. the Chinese avian influenza epidemic characteristics, prevention and control measures. 2006. Feb 5. Available from http:// www.wxhealth.com/qlg/q43.htm.Chinese.
12. Perez DR, Sorrell EM, Donis RO. Avian influenza: an omnipresent pandemic threat. Pediatr Infect Dis J. 2005. 24(11 Suppl):S208–S216.
13. Luo RP, Zhu YM, Xu ZY, Gao JP, Yu SJ. Report of the first human case of H5N1 avian influenza pneumonia in Human, China. Zhonghua Er Ke Za Zhi. 2006. 44:342–345. Chinese.
14. Yu HJ, Chen YX, Shu YL, Li JH, Gao ZC, Hu SX, et al. The first confirmed human case of avian influenza A (H5N1) in Mainland, China. Zhonghua Liu Xing Bing Xue Za Zhi. 2006. 27:281–287.

15. Ministry of Public Health. The bulletin of human avian influenza cases in mainland china. Available from http://news.tom.com/hot/qinliugan/.Chinese.
16. Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO.world health organization. 2006. May 19. Available from http://www.who.int/csr/disease/avian_influenza/country/en/.Chinese.
17. Bridges CB, Kuehnert MJ, Hall CB. Transmission of influenza: implications for control in health care settings. Clin Infect Dis. 2003. 37:1094–1101.

18. Liao DJ, Song B. Review and Epidemiology of Avian Influenza [In Chinese]. J Vet Parasitol. 2004. 12:43–47.
19. Ungchusak K, Auewarakul P, Dowell SF, Kitphati R, Auwanit W, Puthavathana P, et al. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med. 2005. 352:333–340.

20. Zhang CY, Wei JF, He SH. Adaptive evolution of the spike gene of SARS coronavirus: changes in positively selected sites at different epidemic groups. BMC Microbiol. 2006. 6:88.
21. Nash T. Roitt I, Brostoff J, Male D, editors. Immunity to viruses. Immunology. 2001. 6th ed. Harcourt Asia: Pte Ltd;236–237.
22. Sekellick MJ, Carra SA, Bowman A, Hopkins DA, Marcus PI. Transient resistance of influenza virus to interferon action attributed to random multiple packaging and activity of NS genes. J Interferon Cytokine Res. 2000. 20:963–970.

23. Engelhardt OG, Sirma H, Pandolfi PP, Haller O. Mx1 GTPase accumulates in distinct nuclear domains and inhibits influenza A virus in cells that lack promyelocytic leukaemia protein nuclear bodies. J Gen Virol. 2004. 85:2315–2326.

24. Suarez DL, Schultz-Cherry S. Immunology of avian influenza virus: a review. Dev Comp Immunol. 2000. 24:269–283.

25. Lipatov AS, Andreansky S, Webby RJ, Hulse DJ, Rehg JE, Krauss S, et al. Pathogenesis of Hong Kong H5N1 influenza virus NS gene reassortants in mice: the role of cytokines and B- and T-cell responses. J Gen Virol. 2005. 86:1121–1130.

26. Hsieh SM, Chang SC. Insufficient perforin expression in CD8+ T cells in response to hemagglutinin from avian influenza (H5N1) virus. J Immunol. 2006. 176:4530–4533.

27. Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, Kang CY. Alpha-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol. 2005. 175:3309–3317.

28. van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, et al. H5N1 virus attachment to lower respiratory tract. Science. 2006. 312:399.

29. Tumpey TM, García-Sastre A, Taubenberger JK, Palese P, Swayne DE, Pantin-Jackwood MJ, et al. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005. 79:14933–14944.

30. Seo SH, Webster RG. Cross-reactive, cell-mediated immunity and protection of chickens from lethal H5N1 influenza virus infection in Hong Kong poultry markets. J Virol. 2001. 75:2516–2525.

31. Cavanagh LL, Bonasio R, Mazo IB, Halin C, Cheng G, van der Velden AW, et al. Activation of bone marrow-resident memory T cells by circulating, antigen-bearing dendritic cells. Nat Immunol. 2005. 6:1029–1037.

32. Belz GT, Wilson NS, Smith CM, Mount AM, Carbone FR, Heath WR. Bone marrow-derived cells expand memory CD8+ T cells in response to viral infections of the lung and skin. Eur J Immunol. 2006. 36:327–335.

33. Belz GT, Smith CM, Eichner D, Shortman K, Karupiah G, Carbone FR, et al. Cutting edge: conventional CD8 alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2004. 172:1996–2000.

34. Flynn KJ, Riberdy JM, Christensen JP, Altman JD, Doherty PC. In vivo proliferation of naive and memory influenza-specific CD8+ T cells. Proc Natl Acad Sci U S A. 1999. 96:8597–8602.

35. Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influen za antigens promotes CD4 T cell memory generation. J Exp Med. 2005. 202:697–706.

36. Jameson J, Cruz J, Terajima M, Ennis FA. Human CD8+ and CD4+ T lymphocyte memory to influenza A viruses of swine and avian species. J Immunol. 1999. 162:7578–7583.
37. Laudert E, Sivanandan V, Halvorson D. Effect of an H5N1 avian influenza virus infection on the immune system of mallard ducks. Avian Dis. 1993. 37:845–853.

38. Sakai K, Yada K, Sakabe G, Tani O, Miyaji K, Nakamura M, et al. Serological and virological studies of Newcastle disease and avian influenza in slaughter-age ostriches (Struthio camelus) in Japan. J Vet Med Sci. 2006. 68:491–494.

39. Rott R, Klenk HD, Nagai Y, Tashiro M. Influenza viruses, cell enzymes, and pathogenicity. Am J Respir Crit Care Med. 1995. 152:S16–S19.

40. Lipatov AS, Gitel'man AK, Govorkova EA, Smirnov IuA. Changes in biological and physico-chemical properties of avian influenza virus A hemagglutinin H2 during adaptation to a new host. Vopr Virusol. 1995. 40:208–211.
41. Matrosovich M, Zhou N, Kawaoka Y, Webster R. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J Virol. 1999. 73:1146–1155.

42. Bender C, Hall H, Huang J, Klimov A, Cox N, Hay A, et al. Characterization of the surface proteins of influenza A (H5N1) viruses isolated from humans in 1997-1998. Virology. 1999. 254:115–123.

43. Stevens J, Blixt O, Tumpey TM, Taubenberger JK, Paulson JC, Wilson IA. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science. 2006. 312:404–410.

44. Il'iushina NA, Rudneva IA, Varich NL, Lipatov AS, Webster RG, Kaverin NV. Antigenic structure of influenza A virus subtype H5 hemagglutinin: mechanism of acquiring stability to monoclonal antibodies in escape-mutants. Mol Gen Mikrobiol Virusol. 2003. 1:40–45.
45. Chen H, Subbarao K, Swayne D, Chen Q, Lu X, Katz J, et al. Generation and evaluation of a high-growth reassortant H9N2 influenza A virus as a pandemic vaccine candidate. Vaccine. 2003. 21:1974–1979.

46. Chen Z, Matsuo K, Asanuma H, Takahashi H, Iwasaki T, Suzuki Y, et al. Enhanced protection against a lethal infuenza virus challenge by immunization with both hemagglutinin and neuraminidase-expressing DNAs. Vaccine. 1999. 17:653–659.

47. Frace AM, Klimov AI, Rowe T, Black RA, Katz JM. Modified M2 proteins produce heterotypic immunity against infuenza A virus. Vaccine. 1999. 17:2237–2244.

48. Liu W, Zou P, Ding J, Lu Y, Chen YH. Sequence comparison between the extracellular domain of M2 protein human and avian influenza A virus provides new information for bivalent influenza vaccine design. Microbes Infect. 2005. 7:171–177.

49. Renegar KB. Influenza virus infections and immunity: a review of human and animal models. Lab Anim Sci. 1992. 42:222–232.
50. World Health Organization Global Influenza Program Surveillance Network. Evolution of H5N1 avian influenza viruses in Asia. Emerg Infect Dis. 2005. 11:1515–1521.
51. Liu JP. Avian influenza--a pandemic waiting to happen? J Microbiol Immunol Infect. 2006. 39:4–10.
52. Fernandez-Sesma A, Marukian S, Ebersole BJ, Kaminski D, Park MS, Yuen T, et al. Influenza virus evades innate and adaptive immunity via the NS1 protein. J Virol. 2006. 80:6295–6304.

53. Perdue ML. Naturally occurring NS gene variants in an avian influenza virus isolate. Virus Res. 1992. 23:223–240.

54. Chen H, Smith GJ, Li KS, Wang J, Fan XH, Rayner JM, et al. Establishment of multiple sublineages of H5N1 influenza virus in Asia: implications for pandemic control. Proc Natl Acad Sci U S A. 2006. 103:2845–2850.

55. Henley E. The growing threat of avian influenza. J Fam Pract. 2005. 54:442–444.
56. Riedel S. Crossing the species barrier: the threat of an avian influenza pandemic. Proc (Bayl Univ Med Cent). 2006. 19:16–20.

57. Yu SY, Chen Q, Hu GF. Summary of Guangdong provincial seminar on avian influenza and influenza. Di Yi Jun Yi Da Xue Xue Bao. 2005. 25:1587–1588.
58. Normile D. Avian influenza. Chinese Ministry questions bird flu findings. Science. 2005. 309:364.
59. Yang YH. Pay enough attention to human infection with avian influenza virus. Zhonghua Er Ke Za Zhi. 2004. 42:246–247.
60. Chinese Ministry of Public Health. Diagnosis and treatment measures about human influenza A. 2005. Sep 5. Available from http://www.cintcm.com/qinliugan/index_qlg.htm.
61. How to prevent avian influenza of citizen. Shanghai Evening Post. 2004. Jan 29. Available from http://www.china.com.cn/chinese/zhuanti/qlg/487001.htm.Chinese.
62. Wei TB, Huang YT, Li KS. Influenza virus variation and prevention. Chin J Immunol. 2006. 22:16–19. Chinese.
63. Jin NY. Prevalence and preventive control of highly pathogenic avian influenza. Chin J Immunol. 2006. 22:5–12. Chinese.
64. Hang J. My country had successfully advanced development three kinds of new influenza vaccines. Xinhuanet. 2006. June 14. Chinese:
66. Wood JM, Nicholson KG, Stephenson I, Zambon M, Newman RW, Major DL, et al. Experience with the clinical development of influenza vaccines for potential pandemics. Med Microbiol Immunol. 2002. 191:197–201.

67. The Chinese Center for Disease Control and Prevention. The project of human avian influenza's diaginosis and treatment. 2005 Revised edition. Available from http://www.chinacdc.net.cn/n272442/n272530/n273736/n273781/n305111/index.html.
68. Prevention and control of avian influenza in humans in China: achieving the national objectives of the WHO Global Influenza Preparedness Plan Weekly epidemiological record. 2006. 84:105–116. Available from http://www.who.int/wer.
69. Hu X. Speech at the International Conference on Avian Influenza. 2005. Geneva.
70. Leneva IA, Roberts N, Govorkova EA, Goloubeva OG, Webster RG. The neuraminidase inhibitor GS4104 (oseltamivir phosphate) is efficacious against A/Hong Kong/156/97 (H5N1) and A/Hong Kong/1074/99 (H9N2) influenza viruses. Antiviral Res. 2000. 48:101–115.

71. World Health Organization. Avian influenza (bird flu) fact sheet. 2006. Accessed on February 24. At http://www.who.int/mediacentre/factsheets/ avian_influenza/en/index.html.
72. Avian influenza A (H5N1). Weekly Epidemiol Rec. 2004. 79:65–70. (Also available at http://www.who.int/wer/2004/en/wer7907.pdf. (Accessed on February 24, 2006).
73. Chotpitayasunondh T, Ungchusak K, Hanshaoworakul W, Chunsuthiwat S, Sawanpanyalert P, Kijphati R, et al. Human disease from influenza A (H5N1), Thailand, 2004. Emerg Infect Dis. 2005. 11:201–209.

74. Aoki FY, Macleod MD, Paggiaro P, Carewicz O, El Sawy A, Wat C, et al. Early administration of oral oseltamivir increases the benefits of influenza treatment. J Antimicrob Chemother. 2003. 51:123–129.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download