Abstract
Purpose
In non-excitable cells, which include parotid and pancreatic acinar cells, Ca2+ entry is triggered via a mechanism known as capacitative Ca2+ entry, or store-operated Ca2+ entry. This process is initiated by the perception of the filling state of endoplasmic reticulum (ER) and the depletion of internal Ca2+ stores, which acts as an important factor triggering Ca2+ entry. However, both the mechanism of store-mediated Ca2+ entry and the molecular identity of store-operated Ca2+ channel (SOCC) remain uncertain.
Materials and Methods
In the present study we investigated the Ca2+ entry initiation site evoked by depletion of ER to identify the localization of SOCC in mouse parotid and pancreatic acinar cells with microfluorometeric imaging system.
Results
Treatment with thapsigargin (Tg), an inhibitor of sarco/ endoplasmic reticulum Ca2+-ATPase, in an extracellular Ca2+ free state, and subsequent exposure to a high external calcium state evoked Ca2+ entry, while treatment with lanthanum, a non-specific blocker of plasma Ca2+ channel, completely blocked Tg-induced Ca2+ entry. Microfluorometric imaging showed that Tg-induced Ca2+ entry started at a basal membrane, not a apical membrane.
The process of cellular Ca2+ signaling involves regulated changes in the concentration of Ca2+ in the cytoplasm ([Ca2+]i) as well as other cellular compartments. A multitude of cellular processes are controlled through Ca2+ signaling and, in turn, a multitude of external cellular signals induce or regulate Ca2+ signaling. Because so many systems respond to or regulate Ca2+ signaling, it is not surprising that dysfunctions of various aspects of Ca2+ signaling pathways underlie several important diseases.1 When Ca2+ signaling is stimulated in a cell, Ca2+ enters the cytoplasm from one of two general sources: it is either released from intracellular Ca2+ stores or enters the cell across the plasma membrane. Both processes often occur simultaneously or sequentially. In all non-excitable cells, and some excitable cells, an important initiating step is the intracellular release of Ca2+ from internal stores by binding of a second messenger to its receptor in the endoplasmic reticulum (ER). Commonly, this messenger is inositol 1,4,5-trisphosphate (IP3),2 but a number of other potential messengers have been discovered in recent years.3,4 Ca2+ entry can be signaled by a variety of processes, including direct activation by surface receptors and activation by a variety of second messengers;5 however, the most commonly observed mechanism of regulated Ca2+ entry in non-excitable cells is a process knows as capacitative Ca2+ entry or store-operated Ca2+ entry (SOCE).6,7 In many non-excitable cells, depletion of intracellular Ca2+ stores by IP3 is the primary mechanism by which cell surface receptors activate Ca2+ influx. This phenomenon, which is termed capacitative Ca2+ entry,8 has been identified in the control of Ca2+ oscillations,9 secretion,10 and enzymatic regulation.11 Despite the wide range of processes in which SOCE is involved, the signal mechanism that couples store depletion to Ca2+ entry has not yet been fully identified.8 The exocrine acinar cells of the pancreas and the parotid gland are classic examples of non-excitable cells whose key physiological activities are known to be dependent on [Ca2+]i signals that involve a component of Ca2+ entry.12,13 To determine the site of initiation of store- operated Ca2+ entry in non-excitable cells, we investigated the initiation site of SOCE in parotid and pancreatic acinar cells. Our results suggest that the commencement site of SOCE has significance for the future study of the physiological importance of SOCE in parotid and pancreatic acinar cells.
ICR strain mice (23 to 28g) were sacrificed by cervical dislocation. Cells were prepared from the parotids and pancreases of ICR mice by limited collagenase digestion as previously described.14 In order to achieve a pure isolation of acinar cells, density gradient centrifugation was performed with Accudenz (Accurate Chemical and Scientific corp., Westbury, NY, USA), and pure acinar cells were confirmed via light microscope.15 After isolation, the acinar cells were resuspended in an extracellular physiologic salt solution (PSS: NaCl, 140mM; KCl, 5mM; MgCl2, 1mM; CaCl2, 1mM; HEPES, 10mM; and glucose, 10mM, titrated to pH 7.4 with NaOH). The osmolality of the extracellular solution (measured with a FISKE 110 osmometer) was 310 mOsm.
Cells were incubated for 40 min in PSS containing 5µM fura 2-acetoxymethyl ester (Teflabs Inc., Austin, TX, USA) with Pluronic F-127 to enhance dye loading. Changes in [Ca2+]i were measured by means of fura 2 fluorescence, with excitation wavelengths of 340nm and 380nm, and an emission wavelength of 510nm at room temperature. Background fluorescence was subtracted from the raw signals at each excitation wavelength prior to calculating the fluorescence ratio, which was as follows: Ratio = F340/F380. The emitted fluorescence was monitored with a CCD camera (Photon Technology International Inc., Lawrenceville, NJ, USA) attached to an inverted microscope. Fluorescence images were obtained at 0.14s intervals.16 The endoplasmic reticulum of the each cell was emptied with 1µM of thapsigargin in a Ca2+ free state then stimulated by 7.5mM external calcium.
In order to find the initiation sites of store-operated Ca2+ entry (SOCE) in mouse parotid and pancreatic acinar cells, we measured [Ca2+]i increase as follows. The initial step to trigger the SOCE was conducted by depletion of intracellular Ca2+ stores. For this purpose, the endoplasmic reticulium (ER) in parotid and pancreatic acinar cells were depleted by 1µM thapsigargin (Tg), an inhibitor specific to sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), in the absence of Ca2+. To confirm whether ER was definitely emptied by Tg, cells were stimulated with 1mM carbachol in a Ca2+ free state after depletion of ER. When a cellular response to carbachol was no longer observed, the cells were incubated with 7.5mM external calcium for 200 to 400 s so as to trigger SOCE. As a result, we confirmed that vacancy of ER is a kind of signal occurring Ca2+ entry (Fig. 1Aa and Ba. n = 5). However, this finding required us to determine whether the late [Ca2+]i increases were accomplished across the plasma membrane. Thus, lanthanum (La3+), a non-specific blocker of plasma Ca2+ channel, was used to elucidate whether Tg-induced Ca2+ increases were indeed from extracellular sources. Both cell types were treated by 1µM Tg to deplete internal Ca2+ stores, and the cells were then treated with 1mM La3+ for 3 min prior to exposure 7.5mM external Ca2+. We found that the Ca2+ input from external Ca2+ had nearly disappeared in both mouse parotid and pancreatic acinar cells (Fig. 1Ab and Bb).
Next, to identify the initiation sites of SOCE, the initiation site of Tg-induced Ca2+ entry was imaged with fura2 fluorescence. The Ca2+ response generated by the depletion of internal Ca2+ store was not initiated at apical membrane (AM), but rather basal membrane (BM) in parotid and pancreatic acinar cells (Fig. 2A and B, n = 5). In addition, the propagation direction of the initiated Ca2+ entry was from the BM to the AM. There were no differences in Ca2+ entry patterns between parotid acinar cells and pancreatic acinar cells: however, the time scores for Ca2+ entry were different between the two cell types. The half time value of Ca2+ entry (T1/2) in parotid acinar cells was 55.38 ± 6.63 sec following exposure to a 7.5mM Ca2+ solution, while the T1/2 in pancreatic acinar cells was 74.28 ± 6.80 sec., suggesting that the Ca2+ entry in parotid acinar cells was faster than that in pancreatic acinar cells.
In the present work, we investigated the location of the initiation site of SOCE in mouse parotid and pancreatic acinar cells, as identification of this site may be helpful to identify the nature of SOCC. We showed that the store-regulated Ca2+ response initiated at BM of mouse parotid and pancreatic acinar cells after ER depletion by Tg and stimulation by external Ca2+. The mechanism by which Tg effectively empties intracellular Ca2+ stores in exocrine gland acinar cells is through its ability to completely suppress the SERCA.7 Moreover, the Ca2+ response from an external source was diminished when cells were treated with La3+ prior to exposure to external Ca2+, suggesting that Tg-induced Ca2+ entry is mediated by SOCE. Therefore, it is a notable discovery that SOCE initiation begins at the BM in the cells, since there are many Ca2+-signaling related proteins in the apical region in exocrine acinar cells.17
Exocrine acinar cells such as salivary gland acinar cells are structurally and functionally polarized between the basal region and apical region.18,19 The receptors for secretagogues are localized in the basal region, whereas the apical region has a high density of secretory granules that are ultimately released by exocytosis at the apical plasma membrane.20 Many studies have shown that the increase in cytoplasmic free Ca2+ is initiated at the apical region and propagated to the basal region through a Ca2+ wave.21-23 Therefore, it is likely that in polarized exocrine cells, other Ca2+ signaling proteins together with SOCC exist in the basal region of the cells. Recently, a strong candidate and regulator of SOCC were reported as Orai1 and stromal- interaction molecule 1 (STIM1), respectively.24,25 Therefore, it will be necessary to investigate the localization of Orai1 and STIM1 in exocrine gland acinar cells.
Our findings are important, as SOCCs play a fundamental role in both the immediate and long- term regulation of cells. It follows that unexpected perturbations in this process may have the potential for pathological outcomes, while planned pharmacological manipulations may find clinical utility in certain disease states. We look forward to increasing the body of information on the role of SOCCs in disease as well as in the therapy of disease.
Figures and Tables
Fig. 1
Measurement of thapsigargin-induced [Ca2+]i increases. A (a) and B (a): parotid and pancreatic acinar mouse cell responses, respectively, after depletion of internal Ca2+ stores with 1µM thapsigargin (Tg) in nominally Ca2+-free media. Cells were stimulated with 1mM carbachol, and then thapsigargin-induced [Ca2+]i increases were measured by exposure to external 7.5mM Ca2+. A (b) and B (b): parotid and pancreatic acinar mouse cell responses, respectively, after the cells were treated with 1mM lanthanum before exposure to external high Ca2+. Each result was the Trepresentative of 5 independent experiments.
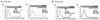
Fig. 2
Measurement of the initiation site of Tg-induced [Ca22+]i increases. (A) and (B) parotid and pancreatic acinar mouse cell responses, respectively. Initiation site of Tg- induced [Ca2+]i increases were imaged with fura2 fluorescence. (C) and (D) The cells were treated with 1mM lanthanum before exposure to external high Ca2+. In each transmission image, red and black circles shows apical and basal pole, respectively. Each result was the representative of 5 independent experiments.

References
1. Missiaen L, Robberecht W, van der Bosch L, Callewaert G, Parys JB, Wuytack F, et al. Abnormal intracellular Ca2+ homeostasis and disease. Cell Calcium. 2000. 28:1–21.
3. Lee HC, Munshi C, Graeff R. Structures and activities of cyclic ADP-ribose, NAADP and their metabolic enzymes. Mol Cell Biochem. 1999. 193:89–98.

4. Petersen OH, Cancela JM. New Ca2+-releasing messengers: are they important in the nervous system? Trends Neurosci. 1999. 22:488–495.
5. Clementi E, Meldolesi J. Pharmacological and functional properties of voltage-independent Ca2+ channels. Cell Calcium. 1996. 19:269–279.

7. Putney JW Jr. Capacitative Calcium Entry. 1997. Austin, TX: Landes Biomedical Publishing;210.
9. Tsien RW, Tsien RY. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990. 6:715–760.

10. Parekh AB, Penner R. Depletion-activated calcium current is inhibited by protein kinase in RBL-2H3 cells. Proc Natl Acad Sci U S A. 1995. 92:7907–7911.

11. Lin WW, Chuang DM. Endothelin- and ATP-induced inhibition of adenylyl cyclase activity in C6 glioma cells: role of Gi and calcium. Mol Pharmacol. 1993. 44:158–165.
12. Tsunoda Y, Stuenkel EL, Williams JA. Characterization of sustained [Ca2+]i increase in pancreatic acinar cells and its relation to amylase secretion. Am J Physiol. 1990. 259:G792–G801.
13. Mertz LM, Horn VJ, Baum BJ, Ambudkar IS. Calcium entry in rat parotid acini: activation by carbachol and aluminum fluoride. Am J Physiol. 1990. 258:C654–C661.

14. Zeng W, Lee MG, Yan M, Diaz J, Benjamin I, Marino CR, et al. Immuno and functional characterization of CFTR in submandibular and pancreatic acinar and duct cells. Am J Physiol. 1997. 273:C442–C455.

15. Xu X, Diaz J, Zhao H, Muallem S. Characterization, localization and axial distribution of Ca2+ signalling receptors in the rat submandibular salivary gland ducts. J Physiol. 1996. 491:647–662.

16. Hong JH, Lee SI, Kim KE, Yong TS, Seo JT, Sohn MH, et al. German cockroach extract activates protease- activated receptor 2 in human airway epithelial cells. J Allergy Clin Immunol. 2004. 113:315–319.

17. Shin DM, Dehoff M, Luo X, Kang SH, Tu J, Nayak SK, et al. Homer 2 tunes G protein-coupled receptors stimulus intensity by regulating RGS proteins and PLCbeta GAP activities. J Cell Biol. 2003. 162:293–303.

18. Segawa A, Sahara N, Suzuki K, Yamashina S. Acinar structure and membrane regionalization as a prerequisite for exocrine secretion in the rat submandibular gland. J Cell Sci. 1985. 78:67–85.

19. Yamamoto-Hino M, Miyawaki A, Segawa A, Adachi E, Yamashina S, Fujimoto T, et al. Apical vesicles bearing inositol 1,4,5-trisphosphate receptors in the Ca2+ initiation site of ductal epithelium of submandibular gland. J Cell Biol. 1998. 141:135–142.

20. Raraty M, Ward J, Erdemli G, Vaillant C, Neoptolemos JP, Sutton R, et al. Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells. Proc Natl Acad Sci U S A. 2000. 97:13126–13131.

21. Toescu EC, Lawrie AM, Petersen OH, Gallacher DV. Spatial and temporal distribution of agonist-evoked cytoplasmic Ca2+ signals in exocrine acinar cells analysed by digital image microscopy. EMBO J. 1992. 11:1623–1629.

22. Nathanson MH, Padfield PJ, O'Sullivan AJ, Burgstahler DV, Jamieson JD. Mechanism of Ca2+ wave propagation in pancreatic acinar cells. J Biol Chem. 1992. 267:18118–18121.
23. Kasai H, Li YX, Miyashita Y. Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell. 1993. 74:669–677.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download