Abstract
Bupivacaine is widely used as a local anesthetic. Central nervous system (CNS) and cardiovascular toxicity are well known side effects. However, there has been no report of bupivacaine-induced myocardial injury. We present a case of bupivacaine cardiac toxicity mimicking an acute non-ST segment elevation myocardial infarction, which was eventually diagnosed as bupivacaine-induced cardiac toxicity without CNS toxicity. As soon as a healthy young woman at a private clinic was given a spinal anesthesia of 6 mg bupivacaine for hemorrhoidectomy, she developed arrhythmia and hypotension. She was transferred to our emergency room. There was an accelerated idioventricular rhythm with ST segment depression on electrocardiogram, coarse breathing sounds with rales on whole lung field and a butterfly sign on the chest radiograph. 2D transthoracic echocardiography (TTE) revealed reduced left ventricle systolic ejection fraction (approximately 27%). There was regional wall motion abnormality of the left ventricle on 2D TTE and the cardiac marker was increased. We diagnosed the patient as having acute non-ST segment elevation myocardial infarction but her impaired cardiac function improved gradually. On the seventh day from admission, there was a complete spontaneous recovery of cardiac function, and coronary angiography revealed a normal coronary artery. Therefore, we firmly believe that bupivacaine directly injures the cardiac cell.
Bupivacaine is widely used as a local anesthetic. Acute and fatal side effects of local anesthetics involve the cardiovascular and central nervous system.1 These side effects usually occur simultaneously after an overdose of local anesthetics or accidental intravascular injections. Cardiovascular side effects can be divided into 2 categories. One is relative to the degree of myocardial conduction depression. The other is relative to negative inotropic action. Bupivacaine produces a concentration-related depression of intra-atrial, A-V nodal, intraventricular conduction and myocardial contractility owing to a fast sodium channel blocking in both nerve and cardiac tissue.2-4 However, there has been no report concerning bupivacaine-induced myocardial injury. We present a case mimicking acute non-ST segment elevation myocardial infarction, which demonstrated a bupivacaine-induced cardiac injury without CNS toxicity.
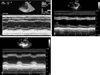
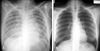
A previously healthy (nonsmoker, non-alcoholic and non-drug abuser) 22-year-old woman underwent spinal anesthesia for an external hemorrhoid operation in a private clinic. She had normal cardiac, respiratory, liver and renal functions with normal laboratory findings on preoperative examination. Vital signs were normal during the operation. Spinal anesthesia was performed with a 23G Quinche spinal needle at the L3-4 interspace in the sitting position. Clear and free flowing cerebrospinal fluid was observed, and heavy bupivacaine 6 mg was administered. Sensory blockade was achieved at S2 dermatome as determined using the pinprick test. The sitting position was maintained for 10 min after injection, then the patient was turned to Jack-knife position and draped. Five minutes after the position change, she complained of chest discomfort, dyspnea and dizziness. Simultaneously, she developed hypotension (80/40 mmHg), ST segment depression on all leads on the electrocardiogram and rales in both lung fields (Fig. 1). The scheduled procedure (hemorrhoidectomy) was cancelled and the patient was transferred to our hospital. Approximately 3 hours after the onset of symptoms she arrived at our emergency room (ER). At that time she had hypotension at 80/36 mmHg, a heart rate of 119 beats per minute, respiratory rate of 24 breaths per minute and a body temperature of 36℃. There was a coarse breathing sound with rales on the whole lung field. The arterial blood gas (ABG) on her arrival with supplemental oxygen (100% venturi mask) was: PaO2: 68.8 mmHg, PaCO2: 41.3 mmHg, pH: 7.29, HCO3: 19.4 mmol/L, BE: -6.7, SaO2: 91.9%. The biomarkers for cardiac necrosis were elevated: creatinine kinase-MB; 15.5 ng/mL (reference range < 6.3 ng/mL) and troponin-I; 2.33 ng/mL (reference range: < 0.2 ng/mL). The brain natriuretic peptide was below 5 pg/mL (reference range < 100 pg/mL). Chest radiograph showed bilateral infiltrates compatible with pulmonary edema (Fig. 2). An echocardiogram performed in the ER showed regional wall motion abnormalities on anteroseptum (akinesia) and severe hypokinesia (whole basal segment) of the left ventricle with decreased global left ventricular (LV) systolic function (LV ejection fraction = 27%) (Fig. 3A). On the basis of her symptoms, signs, laboratory and imaging results, we diagnosed the patient as having acute non-ST elevation myocardial infarction with cardiogenic shock. She was immediately admitted to the coronary care unit and treated with 20 mg furosemide I.V., 13.3 µg/kg/min dopamine, a subcutaneous injection of 40 mg low molecular weight heparin (enoxaparin), 300 mg aspirin and 600 mg clopidogrel orally. Maintenance therapy included a 40 mg subcutaneous injection of enoxaparin every 12 hours, and oral administration of 100 mg aspirin and 75 mg clopidogrel, 40 mg isosorbide dinittrate, 10 mg nicorandil and 40 mg trimetazidine dihydrochloride.
Six hours after initial check-up at the ER, the biomarkers for cardiac necrosis were more elevated: creatinine kinase-MB: 29.4 ng/mL (reference range < 6.3 ng/mL) and troponin-I: 4.22 ng/mL (reference range: < 0.2ng/mL). Twelve hours after the initial ER check-up, the biomarkers for cardiac necrosis showed a slight decline: creatinine kinase-MB: 26.0 ng/mL (reference range < 6.3 ng/mL) and troponin-I: 3.03 ng/mL (reference range: < 0.2 ng/mL). The biomarkers level showed a declining trend. Finally, the creatinine kinase-MB was normalized on hospital day (HD) 4 (creatinine kinase-MB: 1.5 ng/mL; reference range < 6.3ng/mL). On the other hand, troponin-I showed a slightly elevated level on the day of discharge (troponin-I: 0.47 ng/mL; reference range: < 0.2 ng/mL) but this troponin-I level was insignificant.
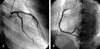
On HD 3, the patient's symptoms improved markedly. Follow up echocardiogram showed an improvement of global and regional wall motion abnormality (LV ejection fraction = 51%) (Fig. 3B). CK-MB and troponin-I were normalized. On HD 6, coronary angiography showed normal coronary artery (Fig. 4). On HD 7, follow up echocardiogram showed no regional wall motion abnormality and normal LV systolic function (LV ejection fraction = 71%) (Fig. 3C). The patient was discharged without complications.
We presented a case of reversible LV dysfunction which occurred immediately after administration of bupivacaine. When the patient arrived at the tertiary hospital, the clinical presentation was a typical feature of cardiogenic shock due to acute coronary syndrome, as she had chest discomfort and dyspnea with elevated cardiac enzyme, typical EKG change and regional wall motion abnormality by echocardiogram.
We diagnosed the patient as having an acute non-ST segment elevation myocardial infarction with cardiogenic shock and pulmonary edema. However, there were several atypical features of acute non-ST segment elevation myocardial infarction in this case. First, the patient was a healthy 22-year-old woman without any family history of coronary artery disease or risk factors. Second, the symptoms occurred immediately after bupivacaine use. Third, the regional wall motion abnormality on the echocardiogram was independent of coronary artery territory.
We considered elevation of sensory blockade level, a viral myocarditis, stress cardiomyopathy, coronary spasm and adverse reaction to the bupivacaine drug as differential diagnoses in the ER. First, elevation of sensory blockade level after spinal anesthesia with bupivacaine did not develop her symptoms and signs (dyspnea, dizziness, bradycardia and hypotension) because she had a tachycardia (heart rate: 119 beats per minute) when her symptom occurred. In addition, her sensitivity was intact when we checked her dermatome level.
Secondly, a viral myocarditis was ruled out because she had no symptoms such as fatigue, fever or myalgia before or after bupivacaine administration. Furthermore, there was no usual tachycardia in a viral myocarditis. In addition, laboratory findings including a WBC count of 5.77 × 109/L (normal range: 4.4-10.0 × 109/L), high sensitive C-reactive protein of 0.04 mg/dL (normal range: < 0.5 mg/dL), and erythrocyte sedimentation rate of 6 mm/h (normal range: 1-20 mm/h) were normal. There was no increased wall thickness which is common in a viral myocarditis on 2D TTE.5 Although the patient had some clinical features similar to those of stress cardiomyopathy, she showed a different 2D TTE finding. There was no preserved basal systolic function, apical akinesia or dyskinesia that are usual in stress cardiomyopathy.6 In addition, abnormal troponin-I is uncommon in patients with stress cardiomyopathy. Therefore, there is little relationship between this case and stress cardiomyopathy.
We were confident that the patient had no coronary spasm because 2D TTE showed regional wall motion abnormality on the anteroseptum (akinesia) and whole basal segment (severe hypokinesia) of the left ventricle, independent of the coronary artery territory. Accordingly, we firmly believe that this patient developed side effects from bupivacaine.
Bupivacaine is related to CNS and adverse cardiovascular effects. CNS side effects usually occur before the cardiovascular signs and symptoms. These include tongue numbness, light headedness, visual disturbances, and muscular twitching. More fatal side effects include convulsions, coma, and respiratory arrest.1 Not only does bupivacaine induce CNS toxicity, but also arrhythmia and myocardial depression due to the blocking of sodium channels in the cardiovascular system.2,3 Bupivacaine significantly decreases the maximum diastolic potential and the action potential amplitude in myocardial tissue, and prolongs the ratio of the effective refractory period to action potential duration.2,7 In that mechanism, bupivacaine produces a depression of myocardial conduction and negative inotropic action.
In a review of the literature, some reports on the adverse effects of bupivacaine were found. Dogs developed hypotension, respiratory arrest, ventricular tachycardia and ventricular fibrillation after bupivacaine administration.8 In the human, 12 volunteers (healthy men) received intravenous injections of bupivacaine, and they developed depression of conductivity and contractility.9 In addition, Coven et al.10 also reported two cases with an accelerated idioventricular rhythm during spinal anesthesia using bupivacaine for caesarean section. Furthermore, Cotileas et al.11 reported a case similar to ours, in which bupivacaine induced myocardial depression and pulmonary edema were described in a healthy young woman with epidural anesthesia for lipoanarrofisis, although she had normal cardiac enzyme levels.
In these reviews, no experience of abnormal CK-MB and troponin-I owing to bupivacaine administration was found, except for this case. We believe that bupivacaine directly damages the myocardium. There is previously published research that supports our opinion. Sztark et al.12 demonstrated that mitochondrial adenosine triphosphate (ATP) synthesis was decreased by bupivacaine in isolated rat heart mitochondria because it acted as an un-coupler between oxygen consumption and phosphorylation of adenosine diphosphate and as an inhibitor of respiration. Consequently, the decrease in cellular ATP resulted in an increased rate of anaerobic glycolysis, which may result in the accumulation of lactic acid and inorganic phosphates from hydrolysis of phosphate esters, cumulating in reduced intracellular pH. Finally, the myocardial cell membrane is injured and intracellular molecules such as cardiac enzymes are released to the blood stream, unless the condition is reversed. Not only does bupivacaine decrease cellular ATP from a molecular biological point of view, but it also decreases coronary blood flow and myocardial oxygen consumption in preparation of isolated perfused guinea pig heart, according to Langendorff.13 Therefore, an increased rate of anaerobic glycolysis may be accelerated and the myocardial membrane injury may be incrementally aggravated.
Based on the above data, we suggest that bupivacaine may induce direct myocardial injury and also release cardiac enzymes in to the blood stream. Therefore, more research regarding bupivacaine-induced myocardial injury in vivo is needed.
Figures and Tables
Fig. 1
(A) Patient's electrocardiogram before surgery. (B) Patient's electrocardiogram when she complained of chest discomfort, dyspnea and dizziness after spinal anesthesia. (C) Patient's electrocardiogram on discharge.
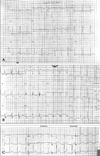
Fig. 3
(A) 2D TTE findings on arrival at ER. 2D Transthoracic echocardiogram (TTE) showed reduced myocardial contractility, diffuse hypokinesia of the left ventricle, especially akinesia of the anteroseptum of the mid-left ventricle and all segments of the left ventricle at the base, mitral regurgitation of grade I/IV and a reduced left ventricular systolic function (ejection fraction: 27%). (B) 2D TTE findings, 3 days after first examination. 2D TTE revealed a normal sized cardiac chamber with improved left ventricle systolic function (ejection fraction: 51%). (C) 2D TTE findings on discharge. Improved heart function (left ventricle ejection fraction: 71%) and morphologically normal heart.
References
1. Knudsen K, Beckman Suurkula K, Blomberg S, Sjovall J, Edvardsson N. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br J Anaesth. 1997. 78:507–514.
2. Arlock P. Actions of three local anaesthetics: lidocaine, bupivacaine and ropivacaine on guinea pig papillary muscle sodium channels (Vmax). Pharmacol Toxicol. 1988. 63:96–104.
3. Clarkson CW, Hondeghem LM. Mechanism for bupivacaine depression of cardiac conduction: fast block of sodium channels during the action potential with slow recovery from block during diastole. Anesthesiology. 1985. 62:396–405.
4. Heavner JE. Cardiac toxicity of local anesthetics in the intact isolated heart model: a review. Reg Anesth Pain Med. 2002. 27:545–555.
5. Matsouka H, Hamada M, Honda T, Kawakami H, Abe M, Shigematsu Y, et al. Evaluation of acute myocarditis and pericarditis by Gd-DTPA enhanced magnetic resonance imaging. Eur Heart J. 1994. 15:283–284.
6. Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005. 352:539–548.
7. Moller R, Covino BG. Cardiac electrophysiologic properties of bupivacaine and lidocaine compared with those of ropivacaine, a new amide local anesthetic. Anesthesiology. 1990. 72:322–329.
8. Feldman HS, Arthur GR, Pitkanen M, Hurley R, Doucette AM, Covino BG. Treatment of acute systemic toxicity after the rapid intravenous injection of ropivacaine and bupivacaine in the conscious dog. Anesth Analg. 1991. 73:373–384.
9. Scott DB, Lee A, Fagan D, Bowler GM, Bloomfield P, Lundh R. Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth Analg. 1989. 69:563–569.
10. Coven G, Arpesella R, Ciceri M, Preseglio I, Cardani A. Accelerated idioventricular rhythm during spinal anesthesia for cesarean section. Int J Obstet Anesth. 2003. 12:121–125.

11. Cotileas P, Myrianthefs P, Haralambakis A, Cotsopoulos P, Stamatopoulou C, Ladakis C, et al. Bupivacaine-induced myocardial depression and pulmonary edema: a case report. J Electrocardiol. 2000. 33:291–296.

12. Sztark F, Malgat M, Dabadie P, Mazat JP. Comparison of the effects of bupivacaine and ropivacaine on heart cell mitochondrial bioenergetics. Anesthesiology. 1998. 88:1340–1349.

13. Tanz RD, Heskett T, Loehning RW, Fairfax CA. Comparative cardiotoxicity of bupivacaine and lidocaine in the isolated perfused mammalian heart. Anesth Analg. 1984. 63:549–556.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download