Abstract
Marchiafava-Bignami disease (MBD) is a fatal disorder characterized by demyelination of the corpus callosum. MRI, suggestive of corpus callosum demyelination with associated white matter involvement in both cerebral hemispheres, indicates a diagnosis of MBD. In this case, MR diffusion-weighted findings taken at an acute stage of MBD revealed lesions not only in the corpus callosum but also in the cerebral cortex. Lower apparent diffusion coefficient values of the corpus callosum and cortical lesions were associated with poor clinical outcome.
Marchiafava-Bignami disease (MBD) is a rare form of toxic demyelination of the corpus callosum associated with chronic alcoholic consumption. Several MR findings have shown lesions not only in the corpus callosum but also in the hemispheric white matter.1-3 To our knowledge, there have been few case reports with MBD in which diffusion-weighted MRI revealed the lesions.4-6 We report a case of MBD in which diffusion-weighted MRI revealed symmetrical hyperintense lesions in the cerebral cortex (particularly in the frontal region) and corpus callosum. Measured apparent diffusion coefficient values in the corpus callosum and cerebral cortices show restriction of water diffusion.
A 61-year-old man presented with acute onset of altered mental status and seizure. The patient had abused alcohol for 30 years and had been diagnosed with alcoholic liver cirrhosis more than 10 years earlier. His family reported that he had consumed a daily average of 300mL of liquor (Korean Soju 25% proof) for 2 weeks. The patient was in poor physical condition and seemed malnourished. On examination, the patient was confused. Although there was no weakness in his extremities, he demonstrated lack of motor coordination. EEG showed diffuse slow waves of 5-8 Hz without epileptiform discharge. Laboratory test results revealed hyperosmosis of 370 mosmol/L (normal 290 mosmol/L), high blood creatinine (2.1 mg/dL, normal 0.5-1.2 mg/dL), and high blood urea nitrogen (BUN) (40.0 mg/dL, normal 8-23 mg/dL). Sodium and potassium levels were, respectively 146 mmol/L and 4.3 mmol/L. CSF studies revealed no abnormalities except low serum levels of vitamin B12, folic acid, and albumin.
Cranial CT showed diffuse periventricular low density and focal areas of low density in the genu and splenium of the corpus callosum. T2-weighted and diffusion-weighted MR images obtained on admission revealed hyperintense lesions involving the genu and splenium of the corpus callosum. In addition, symmetrical cerebral cortical hyperintense lesions were observed in the bilateral frontal cortex. There was also widespread increased abnormal signal in the subcortical and periventricular white matter of the fronto-parietal lobes (Fig. 1). Apparent diffusion coefficient (ADC) mapping yielded a relatively low ADC value in the callosal and cortical lesions (Fig. 2). T2-weighted and diffusion-weighted images at the level of the pons showed a hyperintensity lesion, suggesting old lacunar infarction (Fig. 3A and B). MBD was diagnosed based on the clinical and imaging features. Although the patient was given high-dose vitamin B complex including 500 mg/day thiamine intravenously for 3 days, he went into a coma. On the 7th day of treatment the patient's family decided to have him discharged from the hospital. He died 3 days later.
Marchiafava-Bignami disease (MBD) is a rare form of toxic demyelination seen most frequently in chronic alcoholics. Although it was initially described in drinkers of Italian red wine, it may be seen rarely in persons ingesting other types of alcoholic beverages and poorly nourished non-drinkers. MBD may present as several different clinical forms: an acute form with severe disorders of consciousness and massive neurological disturbance, often resulting in death, and a chronic form, with interhemispheric disconnection, which may last several years. An intermediate form of acute onset of neurological disturbances followed by regression to the chronic form has also been observed.1-3
The main pathologic change associated with MBD is a degeneration of the corpus callosum with different degrees of damage, from demyelination with preservation of axons to necrosis; but, degeneration can extend to the entire corpus callosum and hemispheric white matter. Occasionally, other structures of the CNS may be involved, such as the optic chiasm and tracts, putamen, anterior commissure, cerebellar peduncle, and rarely, cortical gray matter and subcortical U fibers.7,8
MBD is commonly associated with other manifestations of chronic alcohol abuse such as Wernicke's encephalopathy, central pontine myelinolysis, and Morel's laminar sclerosis. Wernicke's encephalopathy is characterized by lesions involving midline structures such as medial thalami, hypothalami, mammillary bodies, and periaqueductal gray matter. In a few cases of Wernicke's encephalopathy, cortical abnormalities are unusually restricted to the motor and premotor cortices.9 Our patient's MR images revealed symmetrical hyperintensity in the frontal motor and premotor cortices. Therefore, we could not exclude the coexistence of subclinical or mild Wernicke's encephalopathy. At the same time, our patient did not present ophthalmoplegia, nystagmus or ataxia and did not show MR findings typical of Wernicke's encephalopathy, such as symmetrical hyperintense lesions surrounding the third ventricle and aqueduct.
Although cortical involvement in MBD is known from pathological studies, a few radiological reports have mentioned cerebral cortical lesions. Johkura et al. reported cases of MBD with bilateral symmetrical hyperintense lesions in the cerebral cortex (particularly in the lateral-frontal regions) on diffusion-weighted images.5 In their reports, the involved cortical lesions had markedly increased signal intensity on diffusion-weighted images and decreased ADC values on ADC maps. Mean ADC values of the involved cortex ranged from 48.7 ± 9.0 × 10-5 to 57.3 ± 8.9 × 10-5 mm2/sec. Our case showed marked reduction of ADC values of the involved cortex, reaching 48.7 ± 9.1 × 10-5 mm2/sec, which results are compatible with water diffusion restriction (Table 1). Johkura et al. explained that radiological cortical abnormality in a patient with MBD may reflect Morel's laminar sclerosis. In addition, reduced ADC in the cortical lesions suggests that the acute phase of Morel's laminar sclerosis is cytotoxic edema.5 Our patient's MR imaging abnormalities were also seen in the corpus callosum and bilateral frontal cortices. ADC measurements revealed a marked decrease in ADC values within the cerebral cortex and corpus callosum. These results demonstrate that pathologic change in acute MBD is due in part to cytotoxic edema of the cortex, and reflect the differing degrees of damage, from demyelination to necrosis.
A recent report revealed that low ADC values in the corpus callosum and combined cortical lesion are associated with a poor prognosis.6 In our case, the patient presented with mental deterioration and died 10 days after clinical onset. Diffusion-weighted MRI showed a corpus callosum and cortical lesion with low ADC values. As shown in Table 1, the ADC measured values for the involved cortex were as low as the values of the callosum. These results suggest that, in the case of acute MBD, cytotoxic edema affects the cerebrum beyond the corpus callosum. Further, combined cortical involvement might have been the cause of the complicated neurologic deficit. However, restricted diffusion on ADC maps does not seem to correlate to the non-reversibility of the lesions, as observed in stroke.10 Further studies providing detailed clinical and neuroimaging data are needed to confirm our results and to elucidate predictors of clinical course and prognosis.
In conclusion, MR diffusion-weighted imaging is useful for the evaluation of callosal and cortical involvement of acute MBD. Lower apparent diffusion coefficient values of the corpus callosum and cortical lesions were associated with poor clinical outcome.
Figures and Tables
Fig. 1
Axial T2-weighted images (TR 4,000/TE116) show hyperintense lesions in the corpus callosum (A) and along both frontal lobe cortices (D). Diffusion-weighted images (B and E, TR 6000/TE 85, b=1,000sec/mm2) show hypersignal intensity in these regions. At the same level as in image B and E, ADC maps (C and F) depict a decreased ADC value, consistent with restricted water diffusion.
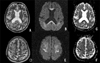
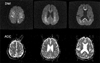
Fig. 2
Comparision of signal intensities on diffusion-weighted images with ADC values on ADC maps. ADC values are markedly reduced in regions with high signal intensity on diffusion-weighted images (regions 1, 2, 7, 8).
References
1. Chang KH, Cha SH, Han MH, Park SH, Nah DL, Hong JH. Marchiafava-Bignami disease: serial changes in corpus callosum on MRI. Neuroradiology. 1992. 34:480–482.
2. Kawarabuki K, Sakakibara T, Hirai M, Yoshioka Y, Yamamoto Y, Yamaki T. Marchiafava-Bignami disease: magnetic resonance imaing findings in corpus callosum and subcortical white matter. Eur J Radiol. 2003. 48:175–177.
3. Arbelaez A, Pajon A, Castillo M. Acute Marchiafava-Bignami disease: MR findings in two patients. AJNR Am J Neuroradiol. 2003. 24:1955–1957.
4. Inagaki T, Saito K. A case of Marchiafava-Bignami disease demonstrated by MR diffusion-weighted image. No To Shinkei. 2000. 52:633–637.
5. Johkura K, Naito M, Naka T. Cortical involvement in Marchiafava-Bignami disease. AJNR Am J Neuroradiol. 2005. 26:670–673.
6. Menegon P, Sibon I, Pachai C, Orgogozo JM, Dousset V. Marchiafava-Bignami disease: diffusion-weighted MRI in corpus callosum and cortical lesions. Neurology. 2005. 65:475–477.
7. Lechevalier B, Andersson JC, Morin P. Hemispheric disconnection syndrome with a 'crossed avoiding' reaction in a case of Marchiafava-Bignami disease. J Neurol Neurosurg Psychiatry. 1977. 40:483–497.
8. Ellison D, Love S, Chimelli L, Harding BN, Lowe J, Vinters HV. Neuropathology. A reference text of CNS pathology. 2004. 2nd ed. Philadelphia: Mosby;489–490.
9. Yamashita M, Yamamoto T. Wernicke encephalopathy with symmetric pericentral involvement : MR findings. J Comput Assist Tomogr. 1995. 19:306–308.
10. Hlaihel C, Gonnaud PM, Champin S, Rousset H, Tran-Minh VA, Cotton F. Diffusion-weighted magnetic resonance imaging in Marchiafava-Bignami disease: follow-up studies. Neuroradiology. 2005. 47:520–524.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download