Abstract
Purpose
We investigated the usefulness of video based, fluoroscopically guided abduction motion analysis of hemiplegic shoulders.
Patients and Methods
Twenty-two stroke patients with Brunnstrom stages 3-4 (Group 1) or 5-6 (Group 2) were enrolled in this study. Patients with shoulder pain and significant spasticity (MAS 2) were excluded. We recorded motion pictures of the abductions of affected and unaffected shoulder joints under an AP fluoroscopic guide. Lateral scapular slide distances (D1: T2-superior angle, D2: T3-scapular spine, D3: T7-inferior angle) were measured at 30°, 60°, 90° during glenohumeral abduction in a captured photographic image. The angles of scapular rotation and trajectory (stromotion) of the humeral head center, relative to the 3rd thoracic spine in the abduction motion were analyzed.
Results
In Group 1, a significant difference was found in the lateral scapular slide distance between the affected and sound sides. However, no significant side to side difference was found in Group 2. Scapular angles in abduction were also increased in Group 1. Patients with a more synergistic movement pattern showed less scapular stabilizing muscle activity and, instead, exhibited a compensatory "shrugging" like motion accomplished by spinal tilting.
Shoulder pain is a common complication of stroke and has been reported to have an incidence between 5% and 84%.1 Clinical presentations of hemiplegic shoulder are variable in nature. Spasticity itself, for example, cannot be judged using only one scale2 because changes in muscle tone differ with time, place, arm position and motion. Moreover, though spasticity is sometimes localized to a specific muscle, it may also be generalized to the whole upper extremity.
The complexity of the clinical manifestations of hemiplegic shoulders suggests that no single diagnostic study is sufficient to define the overall causes of shoulder pathology and current functional status. Accurate quantitative assessment is necessary for monitoring the recovery progress during stroke rehabilitation.
The lateral scapular slide test uses three static testing procedures to evaluate the position of the scapula in relationship to the fixed position of the spine under varying amounts of load. The three positions are with the arm at the side, with the dorsum of hands on the hips, and with the arms abducted at 90°. The inferomedial angle of the scapula in relation to the nearest spinous process is measured on both sides. Side-to-side differences of more than 1.5cm are regarded as the threshold of abnormality, which is most commonly seen with the arms abducted at 90°.3
We modified the lateral scapular slide test by using fluoroscopy. The fluoroscopic lateral slide test is a true dynamic test in that the test positions of interest occur while arm movements are being performed. We recorded all periods during abduction motion and captured the moments of 30°, 60° and 90° of abduction. We then analyzed scapular and humeral movements. Lateral scapular slide distances are measured in captured photographic images.
The aim of the study was to assess the usefulness of abduction motion analysis of the hemiplegic shoulder complex using digital AP fluoroscopy and to design a reliable clinical index of scapular function in hemiplegic patients.
Twenty-two patients who had previously experienced a unilateral cerebrovascular accident for the first time in their life, but retained the ability to abduct the hemiplegic arm, were recruited for this study. All were treated in the inpatient department of Rehabilitation Medicine, Ewha University Mokdong Hospital. The exclusion criteria were (a) shoulder pain during active or passive motion, (b) any shoulder pathology confirmed by musculoskeletal ultrasonography, three phasic bone scan, or by electromyography, (c) severe spasticity of more than MAS (Modified Ashworth Scale) 2, (d) limited passive range of the glenohumeral joint, (e) inability to abduct the hemiplegic shoulder in a sitting position, (f) neglect syndrome, and (g) insufficient cognition to understand the consent form and cooperate with the evaluation.
Patients were subdivided into Groups 1 and 2, according to recovery stage as described by Brunnstrom. Group 1 contained patients between Brunnstrom stages 3 and 4, who initially recovered active voluntary motion by synergy pattern. Group 2 patients were in Brunnstrom stages 5 or 6, and showed isolated voluntary arm motion with a minimal synergy pattern. Informed consent was obtained from all patients.
A digital fluoroscopic device (Philips Tele Diagnost®) was used to assess scapulohumeral motion. The X-ray beam was positioned perpendicular to the coronal plane of the patient and 100 cm from the shoulder joint complex.
Patients were seated so that both the shoulder and pelvis were level on the plate of the fluoroscopy. Arms were abducted from the anatomical position within the scapular plane. Patients were asked not to tilt their spines. After 3-4 practice trials, we recorded motion pictures of the affected and unaffected shoulder joint abductions with an AP fluoroscopic guide. In cases in which a patient was unable to abduct up to 90 degrees, their arms were held at about 60-80 degrees of abduction by the examiner, and the patient was asked to abduct the arm from that position without assistance. During the procedure, the examiner tried to change the position of the participants as little as possible.
We recorded the abduction motion video capture into an AVI file. The analog video sequence was digitized with a time resolution of 30 frames/second. Data processing was performed using a Samsung PC MP20 (P4 2.4GHz, Ram 512MB, HDD 60G) using the Pinnacle Studio version 8 (Studio Deluxe 8, Mountain View, CA, USA).
We replayed the AVI files and measured the distances from specific points in the scapular medial borders to the thoracic spines (Lateral scapular slide distance, D1: T2-superior angle, D2: T3-scapular spine, D3: T7-inferior angle) at 30°, 60°, and 90° of glenohumeral abduction in captured photographic image. Side-to-side differences were compared. The specific point of the spine was the midpoint of both costal facets, which was easily observed by the nearby ribs in the AP view (Fig. 1).
The relationships between each of the clinical variables representing recovery status (Bruunstrom stages, active range of motion) and fluoroscopic measures of scapular function (Difference in lateral scapular slide distance (D) between the sound and affected sides) were of particular interest.
Trajectories (stromotion) of the humeral head centers in relation to the 3rd thoracic spine during abduction motion were analyzed. The centers of the humeral heads were defined as the geometric centers bisected by the long y axis of the humeral shaft and the x axis perpendicular to it.5 The tilting of the spines at abduction were also considered in the analysis of the trajectory and compared with the original trajectories (Fig. 1).
Data are expressed as mean ± standard deviation (SD) or, in tables, the number of cases (expressed as percentages). The data were compared for affected and unaffected sides using the Wilcoxon signed rank test within each group. This test is used in place of the one sample t-test when the assumption of normality is questionable. This test does assume, however, that the population probability distribution is symmetric. Spearman rank correlation coefficients were calculated to establish the relationships between the subjects' recovery status and lateral scapular slide distance differences. We used the Mann-Whitney U test to compare the scapular rotation angles of hemiplegic arms in Groups 1 and 2.
All statistical analyses were performed using SPSS for Windows version 12.0. A significance level of p < 0.05 was used for all comparisons.
Table 1 shows the characteristics of Groups 1 and 2. All data variables are represented by numbers of cases or as means ± standard deviation.
In Group 1, the mean age was 47.3 years (with a range from 16 to 73 years) and the mean number of days from stroke onset was 44.3 ± 61.9. The Brunnstrom stage of proximal upper extremity was 4 in all cases except one. The mean active range of abduction was 79.0 ± 16.6 degrees.
In Group 2, the mean age was 66.8 years (with a range from 48 to 83 years), and the mean number of days from stroke onset was 53.8 ± 54.9. The Brunnstrom stage of the proximal upper extremity was either 5 or 6, and the mean active range of abduction was 123.3 ± 36.6 degrees.
Table 2 shows the differences in the lateral scapular slide distances (D1, D2, D3) in various degrees of shoulder joint abduction. In Group 1, a significant difference was found between the affected and the sound sides in all cases. No specific changes, however, were found in Group 2 between the affected and sound sides. The mean differences are presented in Table 2. The medial borders of the scapular were more closely placed in hemiplegic shoulders than in the sound sides. Placement was closer by 2cm or more. This difference was most prominent in D2 (from T3 to the scapular spine) at 60 degrees of abduction (Table 2).
The Brunnstrom stage correlated more closely with differences in D3 than differences in D1 or D2 (Table 3). The parameters at 90° were more sensitive than those at 30° and 60°. A more significant correlation was found between the Brunnstrom stage and lateral scapular slide distance differences in D1 and D2. The muscle power represented by active range of motion was generally not correlated with lateral scapular slide distance differences, except in D3 at 30° abduction (Table 3).
Table 4 shows the scapular rotation angles at different abduction degrees. The hemiplegic scapular rotation angles were significantly higher than in the sound sides in Group 1 (p < 0.05). In Group 2, the scapular rotation angles were also increased but were only statistical significant at 60 degrees of abduction.
We regarded the T3 spine as the reference point for this study. In imaginary 2 dimensional charts, the trajectory of the humeral head was tracked at 30, 60, and 90 degrees of abduction in captured photographic images. Fig. 2 shows the trajectory of the humeral head centers at 30, 60, and 90 degrees of abduction (from right to left) in each graph. In both groups, as abduction angles increased, trajectories of the hemiplegic humeri were superiorly and medially translated versus the sound sides. After considering the spine rotation angles and reconstructing x and y axes, no significant differences were found the between the two groups in terms of the superior translations of humeral heads. The X value trajectories were moved medially as was observed for arm elevations in Group 1 (p < 0.05).
In studies that have formally assessed the relationships between hemiplegic shoulder pain and multiple clinical variables, some relationships were found between pain and spasticity,6 a reduced range of motion (especially external rotation),7 and anterior subluxation.8
The main clinical characteristics of hemiplegic shoulders are broadly divided as being flaccid or spastic in nature. The flaccid, weak shoulders are prone to subluxation and trauma induced injury such as rotator cuff injury.9 The spastic shoulders, especially those with internal rotator and adductor hypertonicity, cause pain during motion and show limited external rotation. Such patients can be managed with nerve blocks.10
As the humerus abducts or flexes forward, the greater tuberosity of the humerus impinges on the acromion and the coracohumeral ligament, preventing further elevation. The humerus impinges earlier at abduction with the humerus internally rotated, but external rotation permits the greater tuberosity to move behind the coracohumeral ligament and the acromion and further elevation of arm becomes possible. Stroke impairs the coordinated action of the shoulder girdle. Moreover, spasticity and synergy patterns of movement interfere with the mechanism preventing impingement.11
Joynt hypothesized that subacromial impingement is a mechanism of shoulder pain and observed improved symptoms after a subacromial lidocaine injection in about half of his 28 patients.12 The higher incidence of rotator cuff and biceps problems in hemiplegia, and the somewhat better result of subacromial injection or massage in these patients, raises the possibility that impingement may also play a role in the pathogenesis of pain.12-16 Shoulder impingement syndrome was found to be related to rotator cuff insufficiency, posterior capsular tightness, glenohumeral instability, scapular motion disorders or kyphotic deformity of the spine.16 All of these factors are somewhat related to hemiplegic clinical manifestations.
Functional scapular abnormalities have been emphasized to be a cause of shoulder impingement.4 Kibler evaluated abnormal scapular motion in shoulder impingement syndrome by lateral scapular slide test.3 Yoon et al. found this difference in the hemiplegic shoulder and correlated it with clinical findings such as subluxation and pain. They evaluated patients, however, in the resting position only.17
Digital fluoroscopic motion analysis is a useful tool for evaluating the biomechanical properties of hemiplegic shoulders. Almost all stroke rehabilitation centers are equipped with this system for dysphagia evaluation (video fluoroscopic swallowing studies). We were able to clearly draw the medial border of the scapula using this technique, whereas it was hardly visible by simple radiography. In addition, we were able to use the digital system to obtain pictures of the movements required even in non-cooperative cases, and to adjust brightness, contrast, and size. Most importantly, true dynamic motion was visualized using moving pictures, rather than images of static situations. In terms of gait analysis, simple observations of abduction motion videos provide valuable information to the experienced clinician. Moreover, development of this system should further increase its reliability.
Patients in Group 1 had less coordinated scapular stabilization muscle function and much greater lateral scapular slide distance differences. Scapular positions were upwardly rotated and retracted in Group 1. Moreover, this was lessened after motor function recovery, i.e., as Brunnstrom stage increased.
Scapular rotation at abduction is mainly achieved by the upper trapezius and anterior serratus muscles. The middle and lower trapezius is recruited later at above 90 degrees of abduction.18 In the flaccid stage, the glenoid fossa tilts inferiorly because of weakened rotator cuff and scapular stabilization muscles.19,20 After gaining some abduction strength, however, the uncoordinated agonistic and antagonistic actions of the scapular stabilization muscle increase the scapular rotation angle and the glenoid fossa face superior. In impingement syndrome, the scapular rotation angle is also increased in the symptomatic side.3 In the present study, mean scapular angles versus the sound side was significantly higher at Group 1. Group 2 patients also showed increased scapular rotation angles versus the sound sides, but this was not significant except at 60 degrees of abduction, probably because of the small number of patients.
When a hemiplegic patient abducts the affected arm, we usually observe a compensatory shrugging motion. The trajectory of the humeral head center was superiorly translated during this action and was mainly achieved by a tilting motion of the spine. In Group 1 patients, the humeral head translated medially, probably by synergistic flexor and adductor activity in abduction, whereas in Group 2 patients, humeral head trajectory was closely matched by that of the sound side (Fig. 2).
A complex variety of physical changes are associated with hemiplegic shoulder pain. These include weakness, spasticity, synergistic patterns of movement, sensory deficit, and cognitions and perceptions. Limitations of the study are ultimately caused by variable clinical manifestation and the relative lack of subjects necessary to control for these factors.
In the present study, we focused primarily on weak and uncoordinated shoulder girdle muscle function in the absence of significant spasticity or pain. It was hoped that this would simplify results, make them more reliable and exclude the bias that would be introduced by a wider range of clinical characteristics. Otherwise, we could not explore the impact of severe spasticity and painful pathology on shoulder biomechanics.
Patients in Group 2 were much older than those in Group 1 (Table 1), but it appears unlikely that this age difference could have influenced results more than the current recovery status of impairment caused by stroke. The authors already excluded patients with limited range of motion from the shoulder pathology.
We could not compare the lateral scapular slide distance (D) in overhead elevations in terms of the degree to which impingement actually occurs. Moreover, more than half of the patients needed some assistance to elevate their arm up to 90° degree. Assisted motion might alter resting joint position and any subsequent movement patterns.
In the present study, all the pictures were taken in the coronal plane rather than the scapular plane for technical convenience, as it allowed visualization in the right sitting posture and precise side to side comparisons of the lateral scapular slide distances (D). The motion of the shoulder joint in the coronal plane is, however, complex, and is thus difficult to analyze because of interplay between the glenohumeral, scapulothoracic, acromioclavicular, and sternoclavicular joints.5 In order to popularize this fluoroscopic examination system, the developments of more reliable clinical markers of hemiplegic shoulder impingement and scapular function are required. In addition, confirmation of our findings is required in the scapular plane.
There was a small group of patients (2 out of 13) from Group I (Brunnstrom stage 3-4) who actually showed a wider range of abduction than some patients from Group 2 (Brunnstrom stage 5-6). They had considerable abduction muscle power, but their recovery stages did not subsequently improve. These patients had a greater lateral scapular slide distance asymmetry and an increased scapular rotation angle due to the uncoordinated movement of the shoulder girdle muscles. It is not helpful, and is probably harmful, for a patient that has synergistic movement patterns to do repetitive over head exercises, although they have enough power to elevate their arm to the shoulder level. Their exercise program should follow the protocol that does not cause impingement such as exercise in a supine position rather than in a sitting position.
In summary, lateral scapular slide distance difference is negatively correlated to the Brunnstrom stage but not to a greater degree than the active abduction range of motion which also failed to show a statistically positive correlation. More sensitive markers for scapular function are parameters at 90° and those measured in the inferior angle of the scapula (D3). Caution must be used, however, in interpreting lateral scapular slide distance difference at 90° in patients who cannot actively elevate their arm to this level.
In conclusion, we found that the devised method of fluoroscopic-guided shoulder abduction motion analysis provides a useful means of evaluating hemiplegic shoulder biomechanics and that the results of this new method correlate well with the recovery stage. Patients with more synergistic movement patterns showed larger lateral scapular slide distance differences and scapular rotation angle differences compared to measurements of the sound sides. This was probably due to uncoordinated scapular stabilization muscle activity. The compensatory "shrugging" like motion at hemiplegic abduction was not achieved by a true elevation of the humeral head but rather by a tilting of the spine.
Therapy such as strengthening exercises or functional electrical stimulation should be directed to reduce lateral scapular slide distance differences and scapular rotation angles. Moreover, we hope that the effect of therapy can also be determined using the fluoroscopic abduction motion analysis system.
Figures and Tables
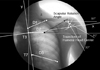 | Fig. 1Lateral scapular slide distance (D), scapular angle, trajectory of the center of the humeral head at 30° abduction in a right hemiplegic patient (image has been flipped for convenience) D, distance from the specific point from the medial border of scapular to the thoracic spine (D1, T2-Superior Angle; D2, T3-Scapular Spine; D3, T7-Inferior Angle) x, and y axes were reconstructed after considering tilting of the spine (white axis), original axis (grey line). |
 | Fig. 2Trajectory of the humeral head center at abduction. The lower two graphs show results after reconstructing the y axis after considering the effect of spinal tilting. |
Table 2
The Mean Lateral Scapular Slide Distance (D) Difference Between Sound and Affected Sides at 30, 60, and 90 Degrees of Abduction

References
1. Turner-Stokes L, Jackson D. Shoulder pain after stroke: a review of the evidence base to inform the development of an integrated care pathway. Clin Rehabil. 2002. 16:276–298.
2. Pandyan AD, Price CI, Rodgers H, Barnes MP, Johnson GR. Biomechanical examination of a commonly used measure of spasticity. Clin Biomech (Bristol, Avon). 2001. 16:859–865.
3. Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998. 26:325–337.
4. Endo K, Ikata T, Katoh S, Takeda Y. Radiographic assessment of scapular rotational tilt in chronic shoulder impingement syndrome. J Orthop Sci. 2001. 6:3–10.
5. Poppen NK, Walker PS. Normal and abnormal motion of the shoulder. J Bone Joint Surg Am. 1976. 58:195–201.
6. Van Ouwenaller C, Laplace PM, Chantraine A. Painful shoulder in hemiplegia. Arch Phys Med Rehabil. 1986. 67:23–26.
7. Bohannon RW, Larkin PA, Smith MB, Horton MG. Shoulder pain in hemiplegia: statistical relationship with five variables. Arch Phys Med Rehabil. 1986. 67:514–516.
8. Cheong JY, Shin HS, Han SJ, Hwang JH, Lee CK. Anterior displacement of humeral head in hemiplegic shoulder subluxation. J Korean Acad Rehab Med. 2002. 26:658–666.
9. Shin JB, Kim SW, Park YS, Kim EH. Causes of the painful hemiplegic shoulder and comparison of the results of radiologic evaluation. J Korean Acad Rehab Med. 2003. 27:293–299.
10. Hecht JS. Subscapular nerve block in the painful hemiplegic shoulder. Arch Phys Med Rehabil. 1992. 73:1036–1039.
11. Cailliet R. Kaplan PE, Cailliet R, Kaplan CP, editors. Musculoskeletal manifestations of hemorrhagic stroke. Rehabilitation of stroke. 2003. 1st ed. Burlington (MA): Elsevier Science;103–128.
12. Joynt RL. The source of shoulder pain in hemiplegia. Arch Phys Med Rehabil. 1992. 73:409–413.
13. Kim SK, Lee KL, Han GS. Ultrasonographic evaluation of the painful hemiplegic shoulder. J Korean Acad Rehab Med. 1999. 23:622–629.
14. Najenson T, Yacubovich E, Pikielni SS. Rotator cuff injury in shoulder joints of hemiplegic patients. Scand J Rehabil Med. 1971. 3:131–137.
15. Lee KM, Lee JH, Park EH. The effect of subacromial massage for hemiplegic shoulder randomized controlled study. J Korean Acad Rehab Med. 2002. 26:385–390.
16. Fu FH, Harner CD, Klein AH. Shoulder impingement syndrome. A critical review. Clin Orthop Relat Res. 1991. 269:162–173.
17. Yoon YS, Jung SS, Lee KA, Kim JH, Lim JT. Assessment of shoulder subluxation using the lateral scapular slide test in hemiplegic patients. J Korean Acad Rehab Med. 2003. 27:819–823.
18. Bagg SD, Forrest WJ. Electromyographic study of the scapular rotators during arm abduction in the scapular plane. Am J Phys Med. 1986. 65:111–124.
19. Calliet R. The shoulder in hemiplegia. 1980. Philadelphia: FA Davis;55–72.
20. Culham EG, Noce RR, Bagg SD. Shoulder complex position and glenohumeral subluxation in hemiplegia. Arch Phys Med Rehabil. 1995. 76:857–864.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download