Abstract
Purpose
To determine and compare the clinical characteristics, visual prognosis and treatment of hemorrhagic polypoidal choroidal vasculopathy (HPCV) with those of hemorrhagic choroidal neovascularization (HCNV) due to age-related macular degeneration (ARMD).
Materials and Methods
Retrospective analysis of 44 consecutive eyes with a submacular hemorrhage comprising more than 50% of the neovascular lesion. Patients were diagnosed as having HPCV or HCNV on the basis of indocyanine green angiography.
Results
Of the 44 eyes with submacular hemorrhage, 26 were classified as HPCV and 18 as HCNV. The baseline patient characteristics were similar for both groups. At the final follow-up the HPCV group had 17 eyes showing visual improvement, four showing maintained vision, and five showing visual deterioration. In contrast, the HCNV group had four eyes showing visual improvement, one showing maintained vision, and 13 showing visual deterioration. Visual acuity of < 0.1 at follow-up was found in 7 (27%) of HPCV eyes and 10 (56%) of HCNV eyes. PDT was performed in 15 HPCV eyes, of which 13 (87%) showed improvement or no change in visual acuity, while only 2 (22%) of the 9 HCNV eyes responded similarly after PDT. Eyes treated with PDT did not have better outcomes compared to eyes that underwent other types of treatment (Fisher's exact test, p > 0.05).
The prognosis for submacular hemorrhage related to age-related macular degeneration (ARMD) is poor, with this disease causing the loss of six lines of vision in 44% of eyes examined.1-3 However, better vision is retained in cases of submacular hemorrhage occurring in polypoidal choroidal vasculopathy (PCV) compared to those occurring in typical ARMD. PCV is a peculiar form of choroidal neovascularization (CNV) that causes persistent recurrent serosanguinous leakage in the macula,4-9 making the retinal manifestation of PCV similar to that of CNV. Submacular hemorrhage and hemorrhagic pigment epithelial detachment (PED) observed in PCV also occur in neovascular ARMD.8 Histopathological study of visible polypoid structures revealed fibrovascular complexes similar to CNV.10 It has been reported that compared to neovascular ARMD, the clinical course of PCV is slower, the prognosis is better, and visual acuity over 0.25 is maintained in most cases.6 The incidence of PCV in Asia has been shown to be as high as 23% in presumed ARMD patients,7 and subretinal or vitreous hemorrhage occurs in 30-64% of PCV cases.4-7
The present study investigated PCV as a cause of submacular hemorrhage, and sought to clarify its clinical characteristics in order to establish a treatment principle. In addition, the study compared the course and treatment outcome of submacular hemorrhages caused by PCV and ARMD.
Forty-four consecutive patients identified with submacular hemorrhage during a three-year period (June 2001 to May 2004) were retrospectively analyzed. The inclusion criteria were that the area of the submacular hemorrhage was greater than 50% of the total area of the CNV or PCV lesion, and that the shortest diameter of the submacular hemorrhage was greater than one disc diameter (DD), evaluated by color fundus photographs. Patients who presented to the clinic one month after the decrease of visual acuity were excluded from this study. Also, these criteria excluded patients with idiopathic CNV, myopic CNV, CNV due to angioid streak or inflammation, or hemorrhage related to macroaneurysm, trauma, laser treatment or anticoagulant medication. PCV was diagnosed based on dynamic high speed indocyanine green angiography (ICGA) (Heidelberg Retina Angiograph, HRA), which was performed at the initial visit or after the hemorrhage had been partially absorbed. Based on ICGA results, PCV was defined as the presence of polypoid lesions and an abnormal choroidal vascular network supplying those lesions. PCV was also diagnosed when ICGA revealed polypoid lesions forming a colony or several polyps, even if choroidal vessels were not clearly observed due to submacular hemorrhage. Among patients diagnosed with PCV, fundus examination revealed that subretinal red-orange nodules were observed near the submacular hemorrhage in two patients.
Medical records of patients were reviewed to obtain data on the clinical history and clinical findings of the disease. Patients were then recalled for further assessment. The data included age at presentation, ethnicity, sex, affected eye, the presence of drusen, the macula condition of the unaffected eye, visual acuity, and the size of the submacular hemorrhage. All patients underwent fluorescein angiography (FAG), ICGA and optical coherence tomography (OCT), and these images were used to analyze the submacular hemorrhage, CNV or polypoid lesion and the underlying vascular network. CNV types were identified using FAG.
Visual outcome was determined for both hemorrhagic PCV (HPCV) and hemorrhagic CNV (HCNV) groups. In addition, since photodynamic therapy (PDT) was the method most frequently used to obliterate PCV lesions, outcomes following PDT treatment were compared to outcomes in non-PDT-treated patients. Development of vitreous hemorrhage and change of size in submacular hemorrhage during follow-up and disciform scar formation were analyzed. Unpaired two-sample t-tests and Fisher's exact test were used for data analysis. A p-value of less than 0.05 was considered a significant difference.
Forty-four eyes of 44 patients with submacular hemorrhage were examined. Twenty-six (59%) eyes were diagnosed with HPCV and 18 (41%) eyes with HCNV due to ARMD. Of the 18 HCNV eyes, FAG results showed that 16 were occult CNV and two were classic CNV.
All patients were Korean. The HPCV and HCNV groups were similar in terms of age, gender, distribution of visual acuity and the size of the presenting submacular hemorrhage. At the initial visit, both groups comprised mostly males with a mean age of 63 years. The mean follow-up period was 16 months (range: 3 to 41 months) for the HPCV group and 20 months (range: 5 to 48 months) for the HCNV group. ICGA showed polypoid lesions in the macula of all 26 HPCV eyes, with small peripapillary polypoid lesions additionally found in two cases concurrent to large polypoid lesions on the macular area and in the peripapillary area of two of those eyes. Of the 26 HPCV eyes, 14 (54%) showed colonies of polypoid lesions such that the exact number of lesions could not be counted. At the initial examination, the mean size of the submacular hemorrhage was 3.6 DD in the HPCV group, and 4.8 DD in the HCNV group. Three (12%) of the 26 HPCV subjects and 9 (50%) of the 18 HCNV subjects showed ARMD manifestations such as drusen, RPE detachment and disciform scars in the other eye (Table 1).
At the initial examination, 2 (8%) of the 26 HPCV eyes and 1 (6%) of the 18 HCNV eyes had visual acuities greater than 0.5. Twelve (46%) HPCV eyes and 8 (44%) HCNV eyes had visual acuities between 0.1 and 0.5, and 12 (46%) HPCV eyes and 9 (50%) HCNV eyes had visual acuities less than 0.1. The distribution of initial visual acuities was similar for both groups, as was the mean visual acuity (0.17 for HPCV and 0.10 for HCNV; p > 0.05). At the last follow-up, 8 (31%) HPCV eyes and 1 (6%) HCNV eye had visual acuities greater than 0.5, 11 (42%) HPCV eyes and 7 (39%) HCNV eyes had visual acuities between 0.1 and 0.5, and 7 (27%) HPCV eyes and 10 (56%) HCNV eyes had visual acuities less than 0.1. Final visual acuities between 0.1 and 0.5 were more frequent in the HPCV cases, while final visual acuities less than 0.1 were more frequent in the HCNV cases (Fig. 1). Analysis of the visual acuities recorded at the initial and final clinic visits showed that vision improved in 21 (48%) of the 44 eyes. Among the HPCV cases, 17 (65%) eyes showed improvement, 4 (15%) eyes were unchanged and 5 (20%) eyes showed deterioration. Among the HCNV cases, 4 (22%) eyes showed improvement, 1 (6%) eye was unchanged and 13 (72%) eyes showed deterioration.
Vision outcomes in PDT- and non-PDT-treated patients were determined for both the HPCV and HCNV groups. In the HPCV group, PDT was performed in 15 eyes (58%), while 11 eyes (42%) did not receive PDT. During the initial follow-up period after the diagnosis was made, tPA and gas injection were performed in both PDT- and non-PDT-treated patients. In the PDT-treated group, 10 of 15 eyes (67%) received tPA and gas injection, and 7 of 11 eyes (64%) underwent tPA and gas injection in the non-PDT-treated group. In the HCNV group, PDT was performed in nine eyes (50%), and nine eyes (50%) did not receive PDT. In the HCNV group, tPA and gas injection were administered in 7 of 9 eyes (78%) for the PDT-treated patients and in 7 of 9 eyes (78%) for the non-PDT-treated patients (Table 2). For the HPCV group, 13 of 15 PDT-treated eyes showed improved or maintained vision, while 8 of 11 non-PDT-treated eyes showed improved or maintained vision. These outcomes were statistically similar (Fisher's exact test, p = 0.34). For the HCNV group, 2 of 9 PDT-treated eyes showed improved or maintained vision, while 3 of 9 non-PDT-treated eyes showed improved or maintained vision. Again, these outcomes were statistically similar (Fisher's exact test, p = 0.5; Fig. 2).
During the follow-up period, 7 (27%) HPCV eyes and 9 (50%) HCNV eyes were found to have a vitreous hemorrhage, and an increase in the size of the submacular hemorrhage was detected in 8 (31%) HPCV eyes and 13 (72%) HCNV eyes. At the last follow-up, 2 (8%) HPCV eyes were found to have larger submacular hemorrhages than initially observed, while 5 (28%) HCNV eyes were found to have larger submacular hemorrhages. Twelve (27%) of the total 44 eyes were found to have residual fibrosis or disciform scarring, three being HPCV eyes (which represented 12% of the HPCV eyes) and nine being HCNV eyes (which represented 50% of the HCNV eyes). Nine of these twelve eyes showed vision less than 0.1, suggesting the disciform scar was the major cause of vision deterioration. In terms of complications, retinal detachment was found in one eye, and hemicentral retinal vein occlusion, which was another cause of vision deterioration, occurred in one HCNV eye.
The diagnosis of PCV has become more accurate and expanded with increased interest in PCV, such that re-examination of previously-diagnosed CNV may now result in a new diagnosis of PCV. The incidence of PCV in Asia has been reported to be higher than that in Europe and North America; 100 (23%) of 471 eyes with presumed neovascular ARMD in Japan were found to have PCV,7 and 85% of large hemorrhagic retinal detachments were associated with PCV.11 This study demonstrated that PCV was the major cause of submacular hemorrhage in Koreans. We found that both HCNV and HPCV patients were mostly male and had a mean age of 63 years. However, the proportion of patients showing unilateral involvement differed between the groups, being 88% for HPCV patients and 50% for HCNV patients. Similarly, studies of Japanese and Chinese patients showed that PCV was observed in older males and was unilateral in many cases.5,7,8,12
While PCV and neovascular ARMD show similar clinical patterns, the two diseases have substantially different progression patterns.4,6-8,13 PCV takes a relatively stable long-term course and rarely causes disciform scarring, whereas neovascular ARMD progresses rapidly and frequently causes disciform scarring. In the present study, vision improved in 17 (65%) of the 26 HPCV eyes, while vision deteriorated to less than 0.1 in 10 (56%) of the 18 HCNV eyes, confirming the poorer visual prognosis for HCNV.
Due to a difference in disease progression patterns, the visual outcome for HPCV was better than for HCNV. During the follow-up period, vitreous hemorrhage developed in 16 (36%) eyes and the submacular hemorrhage size increased in 21 (48%) eyes, indicating a high incidence. Such hemorrhages often developed after tPA and gas injection or PDT. Thus, the increasing hemorrhage size cannot solely be explained by the natural course of submacular hemorrhage. Increased hemorrhage size was observed more frequently in HCNV than in HPCV subjects, and there was a large difference between these two groups in terms of hemorrhage absorption. It was found that the increased hemorrhage size in HCNV patients resulted in decreased visual acuity and facilitated the progression of fibrosis, thus acting as a contributing factor to severe visual loss. In contrast, the hemorrhage in HPCV patients appeared to be readily absorbed, possibly due to the closure of the polypoid lesion and an inactive lesion. The intact RPE in PCV patients has been suggested as another prognostic factor for good visual outcome.
Intravitreal gas injection, intravitreal tPA and gas injection, gas injection with mechanical removal of the blood clot, and vitrectomy with subretinal injection of tPA have been introduced as surgical treatments for submacular hemorrhage.14-17 In the present study, intravitreal tPA and gas injection were initially performed to displace the submacular hemorrhage in 31 (70%) eyes, and PDT was performed in 24 eyes (55%) after the hemorrhage was quite absorbed. In this study, both the HPCV group and the HCNV group were divided into PDT-treated patients and non-PDT treated patients in order to determine the efficacy of PDT. However, there are a few limitations in this study; 70% of the patients received tPA and gas injection prior to PDT, combined treatment was performed in small groups, the subjects were not randomized to compare the efficacy of treatment, and the study was performed in a retrospective manner. Nonetheless, this study still provides sufficient evidence for demonstrating the efficacy of PDT, considering that the proportion of patients receiving tPA and gas injection was similar in both the PDT-treated group and the non-PDT-treated group (for HPCV: 67% vs. 64%; for HCNV: 78% vs. 78%), and the effect of gas injection is mainly the displacement of hemorrhage rather than the closure of vessels in PCV or CNV lesions.
It has been reported that closure of the polypoid lesion occurs readily and that abnormal choroidal vascularity is decreased after PDT in PCV,18-20 which is consistent with the current findings (Fig. 3). The proportion of patients with visual acuity maintained or improved after PDT in the HPCV group was higher (13/15) than that in the HCNV group (2/9). In a recent prospective study by Chan and associates, PDT achieved stable or improved vision in 95% of eyes with PCV.21 However, we found that the PDT group did not result in better vision improvement compared to the non-PDT group, and that spontaneous absorption of the submacular hemorrhage occurred in four eyes and natural disappearance of polypoid lesions occurred in two eyes (Fig. 4). It was reported that submacular hemorrhage developed in 22% of subfoveal classic CNV after PDT,22 and TAP and VIP studies revealed four cases with severe vision loss due to subretinal hemorrhage after PDT.23 In the present study, submacular hemorrhage size increased in 5 (33%) of 15 HPCV eyes and in 6 (67%) of 9 HCNV eyes after PDT. It is unknown whether the increase of submacular hemorrhage after PDT is attributable to the influence of tPA and gas injection. At the last follow-up, two of the five HPCV eyes and all six HCNV eyes had vision less than 0.1. Thus, submacular hemorrhage was found to increase after PDT, and the prognosis was poor. This was most likely because only patients with hemorrhagic events were included, and that the vessels with the hemorrhagic patterns are weaker and more immature than the vessels of the exudative patterns, and that Bruch's membrane was already ruptured.
This study found that a substantial number (59%) of Korean patients with submacular hemorrhage who may have been suspected of having CNV were ultimately diagnosed with PCV. The study confirmed that the visual prognosis for HPCV was better than for HCNV. In addition, we found that for both HPCV and HCNV groups, PDT-treated patients did not have better vision outcomes compared to non-PDT-treated patients. It should be noted that the present data were obtained from a retrospective study of a relatively small number of patients. Further study on the pathogenesis of the polypoid structure of PCV and CNV should be undertaken in order to identify optimal treatments.
Figures and Tables
 | Fig. 1Distribution of initial and final visual acuities in hemorrhagic polypoidal choroidal vasculopathy (HPCV) and hemorrhagic choroidal neovascularization (HCNV) patients. |
 | Fig. 2Visual acuity and PDT treatment in hemorrhagic polypoidal choroidal vasculopathy (HPCV) and hemorrhagic choroidal neovascularization (HCNV) patients. |
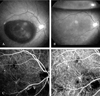 | Fig. 3PCV in a 55-year-old man. (A) Fundus photo showing thick subretinal hemorrhage in the macula of the right eye. The visual acuity was finger counting. (B) Fundus photo taken one day after tPA and SF6 gas injection, showing absorption of the hemorrhage. The visual acuity was 0.4. (C) Early stage ICG angiogram showing an abnormal choroidal vascular network and polypoid lesions connected to abnormal choroidal vessels inferonasal to the macula. (D) ICG angiogram taken three months after PDT. Polypoid lesions can not be detected and visual acuity was 0.8. |
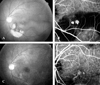 | Fig. 4PCV in a 52-year-old man. (A) Fundus photo showing subretinal hemorrhage and dehemoglobinized blood in the inferior retina involving the macula of the left eye. The visual acuity was 0.1. (B) Early stage ICG angiogram showing two large polypoid lesions. (C) After 16 months without any treatment, absorption of the subretinal hemorrhage is apparent. The visual acuity was 0.7. (D) ICG angiogram showing a fine vascular network of choroidal vessels and absence of polyps. |
References
1. Avery RL, Fekrat S, Hawkins BS, Bressler NM. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina. 1996. 16:183–189.
2. Bennett SR, Folk JC, Blodi CF, Klugman M. Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol. 1990. 109:33–37.
3. Berrocal MH, Lewis ML, Flynn HW Jr. Variations in the clinical course of submacular hemorrhage. Am J Ophthalmol. 1996. 122:486–493.
4. Ciardella AP, Donsoff IM, Huang SJ, Costa DL, Yannuzzi LA. Polypoidal choroidal vasculopathy. Surv Ophthalmol. 2004. 49:25–37.
5. Kwok AK, Lai TY, Chan CW, Neoh EL, Lam DS. Polypoidal choroidal vasculopathy in Chinese patients. Br J Ophthalmol. 2002. 86:892–897.
6. Moorthy RS, Lyon AT, Rabb MF, Spaide RF, Yannuzzi LA, Jampol LM. Idiopathic polypoidal choroidal vasculopathy of the macula. Ophthalmology. 1998. 105:1380–1385.
7. Sho K, Takahashi K, Yamada H, Wada M, Nagai Y, Otsuji T, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003. 121:1392–1396.
8. Uyama M, Wada M, Nagai Y, Matsubara T, Matsunaga H, Fukushima I, et al. Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol. 2002. 133:639–648.
9. Yannuzzi LA, Ciardella A, Spaide RF, Rabb M, Freund KB, Orlock DA. The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol. 1997. 115:478–485.
10. Terasaki H, Miyake Y, Suzuki T, Nakamura M, Nagasaka T. Polypoidal choroidal vasculopathy treated with macular translocation: clinical pathological correlation. Br J Ophthalmol. 2002. 86:321–327.
11. Ahuja RM, Stanga PE, Vingerling JR, Reck AC, Bird AC. Polypoidal choroidal vasculopathy in exudative and hemorrhagic pigment epithelial detachments. Br J Ophthalmol. 2000. 84:479–484.
12. Uyama M, Matsubara T, Fukushima I, Mtsunaga H, iwashita K, Nagai Y, et al. Idiopathic polypoidal choroidal vasculopathy in Japanese patients. Arch Ophthalmol. 1999. 117:1035–1042.
13. Bressler NM; Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-TAP report 2. Arch Ophthalmol. 2001. 119:198–207.
14. Lewis H. Intraoperative fibrinolysis of submacular hemorrhage with tissue plasminogen activator and surgical drainage. Am J Ophthalmol. 1994. 118:559–568.
15. Handwerger BA, Blodi BA, Chandra SR, Olsen TW, Stevens TS. Treatment of submacular hemorrhage with low-dose intravitreal tissue plasminogen activator injection and pneumatic displacement. Arch Ophthalmol. 2001. 119:28–32.
16. Hassan AS, Johnson MW, Schneiderman TE, Regillo CD, Tornambe PE, Poliner LS, et al. Management of submacular hemorrhage with intravitreous tissue plasminogen activator injection and pneumatic displacement. Ophthalmology. 1999. 106:1900–1907.
17. Haupert CL, McCuen BW 2nd, Jaffe GJ, Steuer ER, Cox TA, Toth CA, et al. Pars plana vitrectomy, subretinal injection of tissue plasminogen activator, and fluid-gas exchange for displacement of thick submacular hemorrhage in age-related macular degeneration. Am J Ophthalmol. 2001. 131:208–215.
18. Lee SC, Seong YS, Kim SS, Koh HJ, Kwon OW. Photodynamic therapy with verteporfin for polypoidal choroidal vasculopathy of the macula. Ophthalmologica. 2004. 218:193–201.
19. Rogers AH, Greenberg PB, Martidis A, Puliafito CA. Photodynamic therapy of polypoidal choroidal vasculopathy. Ophthalmic Surg Lasers Imaging. 2003. 34:60–63.
20. Spaide RF, Donsoff I, Lam DL, Yannuzzi LA, Jampol LM, Slakter J, et al. Treatment of polypoidal choroidal vasculopathy with photodynamic therapy. Retina. 2002. 22:529–535.
21. Chan WM, Lam DS, Lai TY, Liu DT, Li KK, Yao Y, et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one-year results of a prospective case series. Ophthalmology. 2004. 111:1576–1584.
22. Gelisken F, Inhoffen W, Karim-Zoda K, Grisanti S, Partsch M, Voelker M, et al. Subfoveal hemorrhage after verteporfin photodynamic therapy in treatment of choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2005. 243:198–203.
23. Arnold JJ, Blinder KJ, Bressler NM, Bressler SB, Burdan A, Haynes L, et al. Acute severe visual acuity decrease after photodynamic therapy with verteporfin: case reports from randomized clinical trials-TAP and VIP report No. 3. Am J Ophthalmol. 2004. 137:683–696.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download