Abstract
Purpose
To test the hypothesis that chronic subdural hematoma (CSDH) enlarges by the causative factors, this study has performed.
Materials and Methods
In 10 patients with CSDH, coagulation factors in venous blood taken at the time of surgery and hematomic contents aspirated from the CSDH were studied, using both laboratory assays and microscopy.
Results
When compared to the range of normal plasma, the hematoma fluids demonstrated a marked reduction in factor II, V, VII, VIII, and X, moderate reduction of factors IX and XI, and slight reduction of factor XII. Activated protein C and antithrombin III levels were decreased. The FDP (Fibrinogen Degradation Product) levels in chronic subdural hematoma were extremely high. The endothelial cells of the macrocapillaries (also called "sinusoid") showed numerous gap junctions between adjacent endothelial cells and a thinness or absence of the basement membrane, suggesting that the macrocapillaries are very fragile and susceptible to bleeding.
Most chronic subdural hematomas (CSDHs) develop in elderly patients after a mild head injury and are surgically curable. Due to recent advances in medicine, the elderly population will continue to increase, and the development of a less invasive treatment for CSDH is desirable. In order for this to occur, it is essential to elucidate the pathogenesis of CSDH. However, the pathogenesis of CSDHs has been controversial for more than a century and still remains obscure. For CSDH development, two theories have been proposed: the osmotic gradient theory and the recurrent hemorrhage from hematoma capsule associated with hyperfibrinolysis theory. The latter hypothesis has been more widely accepted.1
Chronic subdural hematomas are thought to form in the dural border cell layer of the hematoma cavity that is surrounded by outer and inner membranes.2 Whereas there are few blood vessels in the inner membrane, the outer membrane contains many fragile macrocapillaries (also called sinusoidal vessels) that are often the source of repeated multifocal bleeding.2-4 This repeated hemorrhaging from the outer membrane is considered to be a causative factor for progressive enlargement of the hematoma, occurring after a minor head injury.4,5
Chronic subdural hematoma is frequently associated with increased fibrinolytic activity which destabilizes hemostatic clotting, resulting in a recurring hemorrhage from the hematoma capsule.6-8 Coagulation initially proceeds by two separate pathways, the intrinsic and extrinsic systems, which converge to activate the final common pathway causing fibrin formation. The intrinsic system is initiated when blood contacts any surface except normal endothelial and blood cells. The extrinsic system is initiated when tissue thromboplastin activates factor VII.9
We hypothesized that the newly formed fragile sinusoidal vessels were continuously injured, probably by brain pulsation, and that these injuries induced repeated hemorrhaging from the outer membrane, causing the lesions in CSDH to grow slowly and not coagulate. The aim of this study was to test this hypothesis by investigating the expression of coagulopathy in CSDH and growing capillary structure in the outer membrane of CSDH.
Ten patients, six males and four females, with chronic subdural hematoma, including three patients with bilateral hematoma, were studied. The patients ranged from 38 to 72 years of age (mean 59.4 years). Patients with the followings were excluded from our study: coagulopathy, chronic hepatorenal diseases, those on anticoagulation therapy, and those who underwent brain surgery for other lesions. All of the hematomas were completely encapsulated with both outer and inner membranes.
About 10mL of the chronic subdural hematoma was aspirated while under general anesthesia through the small craniotomy or burr hole site in the ipsilateral parietal region before the biopsy of the external membrane. Hematoma fluid samples were centrifuged in plastic tubes, then immediately frozen and stored at -70℃ until analysis. All clotting factors were assessed by the chromogenic substrate method.8 Factor II, V, VII, and X were evaluated by photometric prothrombin time assay and factors VIII, IX, XI and XII by photometric activated partial thromboplastin time (APTT) assay. Activated protein C was measured by the APTT clotting time method10 and antithrombin III by the synthetic chromogenic substrate method (DIAGNOSTICA STAGO, Asnieres Cedex, France).11
We performed the semi-quantitative determination of FDP (fibrinogen degradation product) in plasma and CSDH in all patients by the Latex agglutination method. In this protocol, each patient's plasma and CSDH are tested at two dilutions: 1 : 2 (50 µL patient's sample + 50 µL Reagent 2) and 1 : 8 (50 µL patient's sample + 350 µL Reagent 2). The patient's plasma dilutions and CSDH dilutions were tested, as well as a positive and a negative control in the test-run, so as to have agglutination patterns for comparison. The negative control would not give any agglutination and appear homogeneous. The positive control would give macroscopic clumps.
The outer membrane adhering to the dura mater was carefully obtained from the midportion of the craniotomy in the center of the hematoma, avoiding any mechanical injury to the specimens by the operative procedure.
Ten of the specimens from the external capsule of the hematoma and three of the specimens from the dura mater were fixed in 10% formalin, embedded in paraffin, stained with hematoxylin and eosin (H & E), and prepared for routine light microscopy. The other 10 specimens from the external capsule of hematoma were cut in small pieces and immediately fixed in ice-cold 2% glutaraldehyde in 0.1M cacodylate buffer at pH 7.4 for 2 hours, then postfixed in ice-cold 1% OsO4 in the same buffer for 1 hour. All specimens were then dehydrated with 100% ethanol and embedded in Epon 812. Thick sections for light microscopy were stained with 1% toludine blue, and thin sections for electron microscopy were stained with uranyl acetate and lead citrate. A Nihondenshi JEM-100 B electron microscope (manufactured by Japan Electronics Laboratory, Nakagami-cho, Akishima City, Tokyo, Japan) was used in this study.
Table 1 summarizes the clinical results for all laboratory tests. There were three patients with bilateral CSDH, four patients with CSDH on the left side, and three patients with CSDH on the right side. The hematoma volume ranged from 50 to 250mL (mean 169mL). Factors II, V, VII, VIII, IX, X, XI, and XII were measured in all 13 hematoma fluids. When compared with the normal range for plasma, factor II in the hematoma was normal in one, decreased in 12, and was less than 1% in one hematoma; factor V was normal in two, decreased in 11, and was less than 1% in two hematomas; factor VII was normal in three and decreased in 10; factor VIII was normal in two and decreased in 11, and was less than 1% in three hematomas; factor IX was normal in three and decreased in 10; factor X was normal in one and decreased in 12; factor XI was normal in five and decreased in eight; factor XII was increased in one, normal in nine, and decreased in three.
Activated protein C and antithrombin III were measured in all hematomas. The lower limits in measurement of activated protein C and antithrombin III were 10% and 25%, respectively. Activated protein C was normal in one and decreased in 12, and less than 10% in one. Antithrombin III was decreased in all hematomas and was less than 25% in two.
The FDP levels in patients' blood with CSDH remained nearly normal (1:2 dilution; negative), but the FDP levels in chronic subdural hematoma contents were extremely high (1:8 dilution; positive in all cases).
The dura mater generally contains the two lamellas of the meningeal layer; the compact lamella and loose lamella of the dura mater. The former generally contains tight fibrous tissue and few blood vessels, but the latter contains several blood vessels (Fig. 1).
The outer membrane of the hematoma capsule generally contained numerous macrocapillaries with a wide vascular lumen (Fig. 1). The outer membrane contained the two lamellas. The outer lamella of the outer membrane was shown to be a loose fibrous tissue layer consisted of bundles of collagen fibrils and cellular elements such as fibroblasts, mast cells, a few blood vessels, and other cells. However, the inner lamella contained numerous blood vessels, which were varied in size and form. Most of these vessels were macrocapillaries which are very similar to what Hau et al.12 described as type I capillaries. Scattered red blood cells in the extracellular space, especially within the inner lamella, were found, and this indicated bleeding had occurred within the inner lamella of the outer membrane.
However, when viewed with an electron microscope, their lumens were often extremely wide (≥ 40 µm in diameter), containing several blood cells and consisting of a single layer of flattened endothelial cells with a faint or indistinct basement membrane, but they did not have a complete pericyte or smooth muscle cells investment (Fig. 2).
The endothelial nuclei were elongated or spindle-shaped, with homogenously distributed granular hyperchromatin in the central nucleoplasm, condensing to form a rim near the nuclear membrane. Most of the endothelial cytoplasm has a relatively low electron density. Some short cytoplasmic processes were extruding from the inner surfaces of the endothelium toward the lumen (pseudoprocess). No mitosis was observed. The basement membrane was continuous in some cases and discontinuous in the others. Endothelial gap junctions (0.3-2 µm) were occasionally seen to form at the junction of adjacent endothelial cells. The edges of endothelial gap junctions were outlined by the cellular membranes of endothelial cells. Although we did not detect any deformed erythrocytes within the vascular wall or see any exiting a macrocapillary via a endothelial gap junction, two erythrocytes were seen entering the extravascular space near the endothelial gap junction (Fig. 3).
The subdural space is a closed space. Its outer wall is dura mater that consists of a dense fibrous membrane with poor vascularization, and its inner wall is the vascularized arachnoid with no capillary bed. The inner layer of the dura mater has a very high reaction potential for cellular organization and contains a very fine network of interconnected capillaries. When an acute hematoma is limited to subdural space without arachnoid tear, the hematoma dissects within the layer of dural border cells. All surfaces of all serous cavities normally absorb any foreign material when contact is made. Accumulation of blood, fibrin, and FDP within the subdural space may lead to either cellular organization with resorption of the subdural collection or to the development of a gradually enlarging SDH. Low cerebral counterpressure, a subdural collection that is too large, or a physiological brain atrophy are causative factors for the slow, progressive enlargement of a CSDH.1 The dura border cells usually organizes the hematoma during the second week or later, proliferating and producing a neomembrane (outer and inner membranes), and eventually the hematoma is transfixed by collagen and elastic fibers and sprouting capillaries (sinusoidal vessels). These vessels are fragile and are known to bleed easily. The inner surface of the hematoma develops its own pseudomembrane, separating the clot from the arachnoid. As a result, the neomembrane remains exceptionally vulnerable to traction as long as the proliferative changes continue. Although an increase in collagen would reinforce the neomembrane and culminate in fibrotic healing of the lesion, a vicious cycle may develop in which minor trauma triggers further proliferation of the dural border cells, resulting in the formation of more neomembrane.2-4
Putaman and Cushing13 reported that the external membrane of subdural hematoma is very rich in blood vessels and contains giant capillaries with a lumen 80 µm or more in diameter. These vessels resemble veins, but they do not have a complete pericyte or smooth-muscle cell investment as is usually seen in veins. Many histological investigations of the hematoma membrane have demonstrated the considerable proliferation potential and fragility of the numerous macrocapillaries. The most characteristic clinicopathological aspect of the outer membrane of a CSDH seems to be its tendency to undergo repetitive, multifocal bleeding from the macrocapillaries (also called sinusoids).14,15 The general characteristics of the endothelial cells of the macrocapillaries are a large lumen, attenuated or flattened endothelial cells, scarce cytoplasmic interdigitations, less intimate cellular junctions, gap junctions, and thinness or absence of the basement membrane. These traits suggest that macrocapillaries are very fragile, susceptible to bleeding, and lead to an abnormally high vascular permeability.3,4,16,17 The number and extent of endothelial gap junctions, as large as 0.6 to 8 µm, strongly suggested that they could account for most of the leakage, not only into the tissue of the outer membrane, but also into the hematoma cavity. It is suggested that adjacent endothelial cells may become temporarily separated, allowing erythrocytes, as well as plasma, to escape from the lumen of the macrocapillaries. The mechanism by which the endothelial gap junctions form is not completely understood, but the elevated intraluminal hydrostatic pressure18 or endothelial contractions could induce the separation of adjacent endothelial cells. Perivascular leakage of blood substances occurred easily and repeatedly from the macrocapillaries with such endothelial gap junctions and incomplete basement membrane, and this might contribute to the enlargement of a CSDH.15,18
Watanabe et al.19 demonstrated that the growth content of experimentally induced CSDH was proportional to the thickness of the layer of macrocapillaries and also to the degree of leakage. Yamashima et al.20 stated that the pathogenesis of CSDH enlargement was found to be the result of either direct hemorrhaging of the macrocapillaries or exudation of perisinusoidal edematous fluid into the hematoma cavity, as well as ruptures of small hemorrhagic cavities formed in the outer membranes.
In this study, the external membrane of chronic subdural hematoma is abundant with blood vessels and contains giant capillaries with a lumen 40 µm or more in diameter, wide enough to contain several blood cells, and was lined by several endothelial cells with thin processes. These vessels do not have a complete pericyte or smooth muscle cell. Although no deformed erythrocytes were located within the vascular wall or squeezed out of the macrocapillary through an endothelial gap junction, two erythrocytes were found in the extravascular space around an endothelial gap junction. The following theory is offered to explain the pathogenesis of a chronic subdural hematoma. The outer membrane has many very fragile macrocapillaries with an extensive number of incomplete basement membranes and endothelial gaps, which are susceptible to bleeding when subjected to minor or repeated trauma. Macrocapillaries may develop increased permeability, with consequent leakage of blood substances into the membrane and the hematoma cavity as well.
Putaman and Cushing13 explained that the recurring hemorrhage from the organized outer membrane adjacent to the dura mater caused progressive enlargement of the hematoma because the fibrinogen had been used to build up the neomembrane, followed by the subdural hematoma fluid losing clotting power. However, Ito et al.5 reported that fibrinogen degrades into FDP due to local hyperfibrinolysis, so the subdural hematoma fluid does not usually clot.
In our study, the FDP levels markedly increased in the chronic subdural hematoma when compared to the levels in patients' plasma. Elevated FDP in chronic subdural hematoma suggested enhanced fibrinolytic activity, which could be associated with further bleeding. Fibrin degradation products are well known to have anticoagulation properties and also produce a vasodilator effect. We agree with Ito's hypothesis5 of local hyperfibrinolysis followed by the loss of clotting power in the subdural hematoma fluid. Some degree of fibrinolysis is required to absorb the clot in the subdural space, while abnormally excessive activation of fibrinolysis may well give rise to hemorrhaging from the cavity-lining membrane. Therefore, excessive localized activation of fibrinolysis plays an important role in the etiology of chronic subdural hematoma. The FDP levels in 10 patients with acute subdural hematoma (ASDH, unpublished) were high (positive in eight cases at a 1:8 dilution and in two cases at a 1:2 dilution). If the FDP concentrations in the CSDH and ASDH fluids were measured by a quantitative method, such as hemagglutination-inhibition immunoassay technique, the gradual increase of FDP levels will be shown as an indicator for a switch from being an ASDH to a CSDH, because fibrinolysis takes place in subdural hematoma continuously.
Following injury to blood vessels, platelets begin to accumulate at the damaged area to form the primary hemostatic plug. Plasma coagulation proteins are then activated to initiate secondary hemostasis which then proceeds into the two separate clotting systems.21 The extrinsic clotting system requires factor VII, and the intrinsic clotting system involves factors VIII, IX, XI, and XII. Alternatively, the complex of factor VII, calcium, and tissue thromboplastin released from the extrinsic clotting system can also activate factor IX of intrinsic clotting system. The final common pathway converts factor II to thrombin in presence of factor V, calcium, phospholipids, and factor X. Activated protein C and antithrombin III are most important inhibitors in plasma. Activated protein C inactivates factor Va and VIIIa, and antithrombin III probably causes neutralization of factor Xa, thereby controlling the major amplification step for thrombin formation in both the extrinsic and intrinsic clotting systems.
Our 10 cases demonstrated no basic clotting disorders. The marked reduction in levels of factor VII as well as factors II, V, and X is due to excessive activation of both the extrinsic clotting system and the common final pathway. In contrast, the intrinsic factors did not demonstrate a uniform pattern. Rather, there was a marked reduction in factor VIII, moderate reduction in factor IX and XI, and a slight reduction in factor XII. The lower levels of activated protein C were caused by a depressed inactivation of both factors Va and VIIIa and an accelerated conversion of factors V and VIII to the active form, resulting in excessive consumption. The marked reduction in antithrombin III within the hematoma suggests that the decreased levels of clotting factors were caused by rapid consumption, thereby reflecting excessive coagulation. Therefore, fewer intrinsic clotting factors were used when compared to the extrinsic clotting factors. These results demonstrate that the excessive activation of hematomic coagulation was predominantly via the extrinsic clotting system. Therefore, the basis of chronic SDH growth is the recurrent hemorrhage caused by defective clot formation within the hematoma capsule. The regulatory mechanisms for both coagulation and fibrinolysis are depressed in hematomas. Our results strongly suggest that excessive activation of both coagulation and fibrinolysis are important in the progressive enlargement of chronic SDH.
In addition to hyperfibrinolysis, the hematoma cavity may be subjected to the transmitted pulsation variations produced by continuous brain pulsation and changes in head position.22 This transmission may be accentuated because of the liquid volume of the CSDH trapped within the closed intracranial space surrounding the outer and inner membranes. The transmitted pulsation variations may compress and decompress the sinusoidal vessels found in the outer membrane of the CSDH, and these morphologically fragile vessels may suffer repeated injury.
We performed the small craniotomy or a burr hole at the parietal area and removed the hematoma while taking a biopsy of the outer membrane at the same time. This procedure mitigated the sinusoidal vessel injury by reducing the transmitted pulsation variations in the hematoma cavity. However, if this hypothesis is correct, the creation of a burr hole and continuous drainage should be effective in treating almost all patients with CSDH. The outcome of treatment, such as closed-system drainage, was satisfactory in our all cases. Therefore, we believe that the transmitted pulsation variations may cause sinusoidal vessel injury.
We found that such continuous or intermittent hemorrhaging is the most important factor to maintaining or enlarging CSDH. The causes are the histological characteristics of macrocapillaries, excessive activation of both coagulation and fibrinolysis, and transmitted pulsations.
Figures and Tables
Fig. 1
Photomicrographs of the dura mater. (A) The Compact lamella contains the tight fibrous tissue with fibroblasts, collagen of the meningeal layer(a), the loose lamellas contains the loose fibrous tissue with a few blood vessels(b). (B) Photographs of the outer membrane of subdural caopsule. Note the connective tissue proliferation at the outer side of the outer membrane (compact fibrous tissue, a) and multiple neo-vascular sinusoidal channels overlying fresh red blood cells (loose fibrous tissue; vascular layer, b) ×100.

Fig. 2
Electron micrograph of the external membrane of a subdural hematma showing a macrocapillary. Its lumen is as large as 40m in diameter with erythrocytes (Ec) and consisted of single layer of flattened endothelial cells with a faint or discontinuous basement membrane. Endothelial cells have hyper-chromatic, large nucleus with a high nucleus/cytoplasm ratio. There are two erythrocytes at the outside of the capillary wall. P, pseudopod-like protrusion; N, nucleus; L, lumen (×5,000).
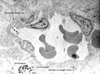
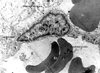
Fig. 3
Electron micrograph of a portion of a macrocapillary with a endothelial gap junction. The endothelal gap junction is as large as 0.6m in diameter. Basement membrane is discontinuous and faint. Two extravasated erythrocytes can be seen. The endothelial cytoplasm contains many ribosomes. L, lumen; Ec, erythrocyte (×10,000).
References
1. Markwalder TM. Chronic subdural hematomas: a review. J Neurosurg. 1981. 54:637–645.
2. Friede RL, Schachenmayr W. The origin of subdural neomembranes. II. Fine structural of neomembranes. Am J Pathol. 1978. 92:69–84.
3. Sato S, Suzuki J. Ultrastructural observations of the capsule of chronic subdural hematoma in various clinical stages. J Neurosurg. 1975. 43:569–578.
4. Yamashima T, Yamamoto S. How do vessels proliferate in the capsule of a chronic subdural hematoma? Neurosurgery. 1984. 15:672–678.
5. Ito H, Yamamoto S, Komai T, mizukoshi H. Role of local hyperfibrinolysis in the etiology of chronic subdural hematoma. J Neurosurg. 1976. 45:26–31.
6. Ito H, Komai T, Yamamoto S. Fibrinolytic enzyme in the lining walls of chronic subdural hematoma. J Neurosurg. 1978. 48:197–200.
7. Weir B, Gordon P. Factors affecting coagulation. Fibrinolysis in chronic subdural fluid collections. J Neurosurg. 1983. 58:242–245.
8. Dati F, Barthels M, Conard J, Fluckiger J, Girolami A, Hanseler E, et al. Multicenter evaluation of a chromogenic substrate method for photometric determination of prothrombin time. Thromb Haemost. 1987. 58:856–865.
9. Kawakami Y, Tanimoto T, Shimamura Y. Coagulopathy in chronic subdural hematoma. Neurol Med Chir (Tokyo). 1991. 31:32–36.
10. Martinoli JL, Stocker K. Fast functional protein C assay using Protac, a novel protein C activator. Thromb Res. 1986. 43:253–264.
11. Scully MF, Kakkar VV. Methods for semi micro or automated determination of thrombin, antithrombin, and heparin cofactor using he substrate, H-D-Phe-Pip-Arg-p-nitroanilide-2HCL. Clin Chim Acta. 1977. 79:595–602.
12. Hauw J, Berger B, Escourolle R. Electron Microscopic study of the developing capillaries of human brain. Acta Neuropathol(Berl). 1975. 31:229–242.
13. Putamen J, Cushing H. Chronic subdural hematoma. Its pathology, its relation to pachymeningitis hemorrhagica and its surgical treatment. Arch Surg. 1925. 11:329–393.
14. Rhodin JA. Ultrastructure of mammalian venous capillaries, venules, and small collecting veins. J Ultrastruct Res. 1968. 25:452–500.
15. Yamashima T, Yamamoto S, Friede RL. The role of endothelial gap junctions in the enlargement of chronic subdural hematomas. J Neurosurg. 1983. 59:298–303.
16. Yamamoto T, Sato S. Electron microscope studies on the vessel wall and the leukocyte emigration. Arch Histol Jpn. 1966. 27:297–310.
17. Scotti G, Terbrugge K, Melancon D, Belanger G. Evaluation of the age of subdural hematomas by computerized tomography. J Neurosurg. 1977. 47:311–315.
18. Rowley DA. Venous constriction as the cause of increased vascular permeability produced by 5-hydroxytryptamine, histamine, bradykinin, and 48/80 in the rat. Br J Exp Pathol. 1964. 45:56–67.
19. Watanabe S, Shimada H, Ishii S. Production of clinical form of chronic subdural hematoma in experimental animals. J Neurosurg. 1972. 37:552–561.
20. Yamashima T, Shimoji T, Komai T, Kubota T, Ito H, Yamamoto S. Growing mechanism of chronic subdural hematoma-light and electron microscopic study on outer membranes of chronic subdural hematoma (author's transl). Neurol Med Chir(Tokyo). 1978. 18:743–752.
21. Handin RI. Braunwald E, Fauci A, Kasper DL, Hauser S, Longo DL, Jameson JL, editors. Bleeding and thrombosis. Harrison's Principles of Internal Medicine. 2005. 16th ed. New York: McGraw-Hill;337–343.
22. Murakami H, Hirose Y, Sagoh M, Shimizu K, Kojima M, Gotoh K, et al. Why do chronic subdural hematomas continue to grow slowly any coagulate? Role of thrombomodulin in the mechanism. J Neurosurg. 2002. 96:877–884.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download