Abstract
Purpose
Cervical cancer is a major women's health problem in the world today. The purpose of this study was to estimate the incidence and mortality rates and to investigate risk factors for cervical cancer in Korean women.
Materials and Methods
Reproductive factors, cigarette smoking, as well as the risk of incidence and death from cervical cancer were examined in a 12-year prospective cohort study of 475,398 Korean women aged 30 to 95 years who received health insurance from the National Health Insurance Corporation and who had a medical evaluation in 1992. Relative risks (RR) and 95% confidence intervals (CI) were calculated using the Cox proportional hazards model after adjusting for age, body mass index, cigarette smoking, alcohol use, menarche, parity, and Papanicolaou test status.
Results
This study showed that the RR of death due to cervical cancer among current smokers was two times higher compared with non-smokers (RR = 2.00; 95% CI, 1.23-2.91). In addition, the RR of death due to cervical cancer among all women who smoked ≥ 10 cigarettes/day was 2.4 times higher than the RR among women that had never smoked. More interestingly, those who had never been screened by Papanicolaou smears had twice the risk of death due to cervical cancer (RR = 2.00; 95% CI, 1.37-1.81).
Cervical cancer is the rapid, uncontrolled growth of severely abnormal cells in the cervix, which is the lower part of the uterus that opens into the vagina.1,2 This preventable disease kills an estimated 288,000 women every year.3 The highest incidence rates have been observed in sub-Saharan Africa, Melanesia, Latin America, the Caribbean, Southcentral Asia, and Southeast Asia. In Korea, cervical cancer is still the most common gynecologic malignancy despite the fact that widespread cytological screening has reduced the incidence of this cancer.4,5 The incidence of cervical cancer (age-standardized rate) in Korea was estimated at a rate of 18.0 out of 100,000 women. Of the diseases with the highest incidence in Korean women, cervical cancer ranked third after stomach and breast cancers.6
Many previous studies have explored the risk factors associated with cervical cancer. These risk factors include menarche, high parity, an early age at first intercourse, multiple male sex partners, a history of sexually transmitted diseases, smoking, certain nutritional deficiencies, and low socioeconomic status.7-9
The present study, which is the largest population-based cohort study of cervical cancer conducted to date in Korea, provides a unique opportunity to examine the relationship between reproductive factors, cigarette smoking, and Papanicolaou smears with the incidence and mortality associated with cervical cancer.
The National Health Insurance Corporation (NHIC), previously known as the Korea Medical Insurance Corporation, provides health insurance to government employees, teachers, and their dependents. Of the entire Korean population (approximately 43.7 million in 1992), 10.7% were insured by this organization in 1992, including 1,297,833 workers and 3,364,605 dependents. All workers were required to participate in biennial medical examinations. In 1992, 94% of the insured workers had complete examinations.
The present cohort includes 482,618 Korean women aged 30 to 95 years who received health insurance from the NHIC and who had a medical evaluation in 1992. Women were excluded if they had a history of any form of cancer, diabetes before the start of follow-up, or Papanicolaou smear results with missing variables. Therefore, the remaining 475,398 subjects were included in the final analysis. Data on the Papanicolaou smears was available for 271,593 (57.1%) of the subjects.
The NHIC biennial examinations followed a standard procedure and were conducted by local medical staff at each local hospital in Korea. This dataset consisted of questionnaires that were completed in 1992, 1993, 1994, and 1995. These questionnaires included information on past and present illnesses as well as menstrual and reproductive factors. Questions on reproductive factors included age at menarche, age at first delivery, number of offspring, menopause, and age at menopause. Lifestyle related factors including smoking habits, alcohol intake, and exercise were also described. Trained staff reviewed completed questionnaires and entered the data.
In addition, this dataset included information taken from biennial medical examinations conducted between 1992 and 2004. The examinations gained data on Papanicolaou smear results, height, weight, and blood pressure (BP) status. At each medical examination, medical staff measured body mass index (BMI), which was calculated as weight (kg) divided by height (m2), and blood pressure, with a standard mercury sphygmomanometer or automatic manometer when the individual was seated. A blood sample from each patient was obtained to determine fasting serum glucose (FSG) levels. Our study was approved by the Institutional Review Board at Yonsei University.
The principal outcome variables were incidence of and mortality from cancer of the cervix. Patient outcomes from cervical cancer cases were based on national cancer registry data and hospitalization records. Although Korea has a national cancer registry, the reporting to this database was not complete during the time of follow-up for our study. Thus, hospital admission files were used to identify a first admission event for cervical cancer. An incident of cancer was coded based on either a positive report from the national cancer registry or on a hospital admission for a cancer diagnosis.
An outcome of mortality was ascertained from the cause of death denoted on the death certificate. A computerized search of death certificate data from the National Statistical Office in Korea was performed using the unique identification number assigned at birth. Causes of death were assigned at the hospitals by trained abstractors. Cervical cancer was classified according to the 10th revision of the International Classification of Disease: Malignant neoplasm of the cervix (ICD-10, C50).
Chi-square and ANOVA tests were used to analyze statistical differences among characteristics of study participants with regard to their Papanicolaou smear results. Study participants were classified into three main groups according to the Papanicolaou test status: 1) unscreened women, 2) screened women with normal test results, and 3) screened women with abnormal test results. To identify the risk factors of cervical cancer, all participants were included for further analyses.10
Age-adjusted mortality and incidence rates were calculated for groups classified according to smoking status and were directly standardized to the age distribution of the Korean population in 1995.11 Cox proportional hazard regression models were used to compute relative risks (RRs) and 95% confidence intervals (CIs), after adjusting for age and other potential confounding factors.12 A Cox proportional hazard model was used to estimate the risk of each reproductive and behavioral factor as well as the Papanicolaou smear result variables against cervical cancer incidence and death.
The variables for which risk was calculated were: age at menarche (≤ 14, between 15 and 16, and ≥ 17 years old), age at first delivery (no delivery, ≥ 26, between 22 and 25, ≤ 21 years old), menopause status (yes or no), age at menopause, which was defined as the age at which menses had not occurred for at least one year (≤ 49, between 50 and 54, and ≥ 55 years old), and the duration of estrogen exposure, which was defined as the period of years between menarche and menopause (≤ 32, between 33 and 37, and ≥ 38 years).13-15 The other factors taken into consideration were BMI (≤ 18.49, 18.5-24.9 and ≥ 25 kg/m2)16 and alcohol consumption (yes or no). Smoking status was categorized into three groups: non-smoker, ex-smoker, and current smoker. In addition, the average number of cigarettes smoked daily was calculated and women were placed in three groups accordingly, 1-9 and ≥ 10 cigarettes/day. All analyses were conducted using SAS statistical software, version 8.2 (SAS Institute Inc, Cary, NC USA). Data were considered statistically significant when p was < 0.05.
Forty-three percent (n = 203,805) of the total number of women were not screened by Papanicolaou smears. The mean age of this group of women was 44 years. The remaining 57% (n = 271,593) of women were screened by Papanicolaou smears (Table 1). Among these screened women, 78,503 (28.9%) women had abnormal results and 193,090 (71.1%) women had normal results. The mean age of women with normal and abnormal test results was 52.5 and 55.6 years, respectively. The mean BMIs for unscreened and screened women were 21.4 and 24.0 respectively (p < 0.0001). Most of the women in this study were nonsmokers. Cigarette smoking was substantially more common in women with abnormal test results compared to women with normal test results. Women with abnormal Papanicolaou smear results were more likely to have had a first delivery and reached menopause at an earlier age than women with normal test results.
The overall incidence rate of cervical cancer among all women was 45.9 per 100,000 women while the mortality rate was 3.4 per 100,000 women (Fig. 1). The highest incidence rate (13.58 per 100,000) was observed in women aged 30-39 years. Women aged 50 years or older had the highest mortality rate due to cervical cancer.
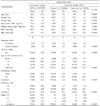
Table 2 shows the multivariate-adjusted relative risks of cervical cancer incidence and mortality. Women who had late menarche (age at menarche was ≥ 17) were at increased risk of cervical cancer compared with women who had early menarche (RR = 1.22, 95% CI, 1.09-1.36 in model 2). Because of the missing variables for parity (99% of the study subjects did not answer to this question), there was no statistical significance for parity as a risk factor of cervical cancer (not shown). Table 2 also shows an increased risk associated with first delivery at an early age, with women ≤ 21 years old at the time of their first delivery having 1.5 times the risk of cervical cancer compared with women whose first delivery occurred at age 26 years or older (RR = 1.56; 95% CI, 1.31-1.86).
Women who had reached menopause had an increased risk of cervical cancer compared to women who had not reached menopause (RR = 1.36, 95% CI 1.19-1.54). Age at menopause was not significantly associated with cervical cancer risk, whereas long-term estrogen exposure was associated with an increased risk. No relationship was found between reproductive factors and risk of death due to cervical cancer. Women with abnormal Papanicolaou smear test results had an increased risk of cervical cancer. More interestingly, those who had never been screened by a Papanicolaou smear also had an increased cervical cancer risk when compared to those who had normal Papanicolaou smear results (RR = 1.12; 95% CI, 1.00-1.25). Women who had never been screened by a Papanicolaou smear had twice the risk of death due to cervical cancer compared to those who had normal results (RR = 2.00; 95% CI 1.37-1.81).
The incidence rate of cervical cancer among women who had never smoked was 45.7 per 100,000 person-years and the mortality rate was 3.1 per 100,000 person-years (Table 3). The incidence rate of cervical cancer among women who had either smoked in the past or currently smoked was lower than women who had never smoked. However, the mortality rate among women who currently smoke was two times that of women who had never smoked. In addition, the relative risk of death due to cervical cancer among current smokers was two times higher compared with those that had never smoked (RR = 2.00; 95% CI, 1.23-2.91). Furthermore, our study showed that the RR of death due to cervical cancer among all women who smoked ≥ 10 cigarettes/day was 2.4 times higher compared to that of women who had never smoked (nonsmokers) (RR = 2.40; 95% CI, 1.34-4.17) (Table 4).
Cervical cancer is a complex and multifactorial disease. This large prospective cohort study of Korean women showed that several reproductive factors including age at menarche, age at first delivery, menopause status, and long-term estrogen exposure were independent risk factors for the development of cervical cancer. The overall mortality rate of cervical cancer among all women in this study was 3.4 out of 100,000 women while the incidence rate was 45.9 per 100,000 women. Several studies on cervical cancer incidence have shown consistently that a young age at first intercourse, a high number of sexual partners, a high parity, cigarette smoking, race, and low socioeconomic status are significant risk factors for cervical cancer.17,18 However, our study dataset did not include variables such as sexual behavior and socioeconomic status.
A study by Peters et al. found some evidence that subjects with short intervals between menarche and the initiation of sexual intercourse had an elevated risk (with relationships stronger than those observed with age at first intercourse considered as a factor alone), but this effect was not confirmed in a later study, which found no evidence of such an effect.17,19 There was a positive association between the risk of cervical cancer and the number of pregnancies, however this risk was significant only when considering incident cases. Many studies have suggested that high parity contributes to the risk of cervical cancer.20 We were not able to research the association between high parity and cervical cancer due to the fact that 99% of the women surveyed did not answer this question. Sriamporn and his colleagues reported that the age at delivery of the first child was weakly associated with risk for incident cases.21 Interestingly, our study showed that age at first delivery was strongly associated with a higher risk of cervical cancer.
In recent years, cervical cancer incidence rates have been increasing among women aged 30-39 years and may reflect, in part, the fact that Papanicolaou smears were previously not used. A study by Misra et al. reported that approximately 30% of all cervical cancer was in patients aged 41 to 50 years old.22 Furthermore, this study showed women who had never been screened by Papanicolaou smear were at twice the risk of mortality due to cervical cancer than those who had normal test results. An inflation factor of 1.67 was applied to estimate the underlying incidence rate in patients without any previous screening, and it gives an annual rate of 27.2 per 100,000 in the UK.23
Cervical screening programs based on regular Papanicolaou smears have been responsible for very significant decreases in both the incidence and mortality due to cervical cancer in all countries that have functional screening programs.24,25 The goal of cervical screening in the United States is to identify and remove significant precancerous lesions in addition to preventing mortality from invasive cancer.26 Rates of cervical cancer in the United States have decreased from 14.2 new cases per 100,000 women in 1973 to 7.8 cases per 100,000 women in 1994. Despite a decrease in the incidence, cervical cancer remains one of the leading causes of death associated with cancer. The Healthy People 2010 target for cervical cancer is a reduction in mortality to 2.0 deaths per 1,000,000 women. Since 1998, the rate has remained near 3.0 deaths per 100,000 women. The United States Preventive Services Task Force (USPSTF) strongly recommends screening for cervical cancer in women who have been sexually active and have a cervix.27
In addition, cigarette smoking has been related to cervical cancer incidence. Most studies show that there is a strong correlation between women with cervical neoplasia and those who currently smoke.28,29 Current smoking (even passive smoking) has been linked to a 2-fold increase in the risk of developing cervical cancer.30,31 In addition, we have found an association between cervical cancer and the average number of cigarettes smoked daily, as well as the length of time a woman had been smoking. The presence of cigarette carcinogens in cervical mucus has been described as a possible biological explanation for the epidemiological association.32,33 The possible mechanisms through which cigarette smoking exerts its effect could involve soluble carcinogens that may have a direct transforming effect on cervical epithelium.
The strengths of this study include its prospective design and large sample size. In addition, it is likely that this cohort is not identical to the whole female population of Korea. Our study has assessed the risk of cervical cancer in terms of both incidence and mortality rates within the same large population. Because of these advantages, we were able to investigate the relationship between a wide range of reproductive factors as well as cigarette smoking and cervical cancer risk in Korean women. The potential limitations of our study result primarily from the use of data collected for clinical purposes and which might therefore tend to lack sufficient information, for our purposes, pertaining to specific reproductive factors. Additionally, many of the health examinations were conducted at different hospitals throughout the country and the laboratory techniques were not standardized.
In conclusion, our study found that late menarche, early age at first delivery, menopause status, and long-term estrogen exposure were independent risk factors for cervical carcinogenesis. Current cigarette smoking was associated with increased risk of death due to cervical cancer. The recent increasing trend in the number of Korean women who smoke could have a serious impact on cervical cancer mortality in the coming years. Our data suggests that the target age group for cervical cancer screening tests should be reconsidered and screening tests should commence in patients at an earlier age. Continued efforts are needed to increase the use of Papanicolaou smears in order to effectively close the gap between the women who have not been screened and the women who have been screened. An increased use of Papanicolaou smears will also reduce cervical cancer mortality as well as the overall burden of cervical cancer among Korean women.
Figures and Tables
Table 2
Relative Risks (RR) of Cervical Cancer Incidence and Mortality by Reproductive Factors and Papanicolaou Test Status among Study Subjects

*Adjusted for age, BMI, cigarette smoking, alcohol drinking, menarche and parity. CI, confidence interval.
†Estrogen exposure duration which was defined as the period of years between menarche and menopause.
‡Papanicolaou test status was adjusted for age, BMI, cigarette smoking, alcohol drinking, menarche and parity.
ACKNOWLEDGMENTS
We thank the staff of the Korean National Health Insurance Corporation who provided the data for this study.
References
1. Elkas J, Farias-Eisner R. Cancer of the uterine cervix. Curr Opin Obstet Gynecol. 1998. 10:47–50.
2. Cole HM. Women's health on the Web. JAMA. 1999. 282:1211–1212.
3. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005. 55:74–108.
4. Jung WW, Chun T, Sul D, Hwang KW, Kang HS, Lee DJ, et al. Strategies against human papillomavirus infection and cervical cancer. J Microbiol. 2004. 42:255–266.
5. Kim IS, Suh I, Oh HC, Kim BS, Lee Y. Incidence and survival of cancer in Kanghwa County (1983-1987). Yonsei Med J. 1989. 30:256–268.
6. Shin HR, Ahn YO, Bae JM, Shin MH, Lee DH, Lee CW, et al. Cancer Incidence in Korea. Cancer Res Treat. 2002. 34:405–408.
7. Kuper H, Boffetta P, Adami HO. Tobacco use and cancer causation: association by tumour type. J Intern Med. 2002. 252:206–224.
8. Beral V, Hermon C, Munoz N, Devesa SS. Cervical cancer. Cancer Surv. 1994. 19-20:265–285.
9. Weiderpass E, Ye W, Tamimi R, Trichopolous D, Nyren O, Vainio H, et al. Alcoholism and risk for cancer of the cervix uteri, vagina, and vulva. Cancer Epidemiol Biomarkers Prev. 2001. 10:899–901.
10. Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SA, Pettinger M, et al. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women's Health Initiative randomized trial. JAMA. 2003. 290:1739–1748.
11. Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005. 293:194–202.
12. Kaneko S, Tamakoshi A, Ohno Y, Mizoue T, Yoshimura T; JACC Study Group. Menstrual and reproductive factors and the mortality risk of gastric cancer in Japanese menopausal females. Cancer Causes Control. 2003. 14:53–59.
13. Green J, Berrington de Gonzalez A, Sweetland S, Beral V, Chilvers C, Crossley B, et al. Risk factors for adenocarcinoma and squamous cell carcinoma of the cervix in women aged 20-44 years: the UK National Case-Control Study of Cervical Cancer. Br J Cancer. 2003. 89:2078–2086.
14. Hinkula M, Pukkala E, Kyyronen P, Laukkanen P, Koskela P, Paavonen J, et al. A population-based study on the risk of cervical cancer and cervical intraepithelial neoplasia among grand multiparous women in Finland. Br J Cancer. 2004. 90:1025–1029.
15. Geerlings MI, Ruitenberg A, Witteman JC, van Swieten JC, Hofman A, van Duijn CM, et al. Reproductive period and risk of dementia in postmenopausal women. JAMA. 2001. 285:1475–1481.
16. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004. 363:157–163.
17. Brinton LA, Hamman RF, Huggins GR, Lehman HF, Levine RS, Mallin K, et al. Sexual and reproductive risk factors for invasive squamous cell cervical cancer. J Natl Cancer Inst. 1987. 79:23–30.
18. Herrero R, Brinton LA, Reeves WC, Brenes MM, Tenorio F, de Britton RC, et al. Sexual behavior, venereal diseases, hygiene practices, and invasive cervical cancer in a high-risk population. Cancer. 1990. 65:380–386.
19. Peters RK, Chao A, Mack TM, Thomas D, Bernstein L, Henderson BE. Increased frequency of adenocarcinoma of the uterine cervix in young women in Los Angeles County. J Natl Cancer Inst. 1986. 76:423–428.
20. Eluf-Neto J, Booth M, Munoz N, Bosch FX, Meijer CJ, Walboomers JM. Human papillomavirus and invasive cervical cancer in Brazil. Br J Cancer. 1994. 69:114–119.
21. Sriamporn S, Parkin DM, Pisani P, Suwanrungruang K, Pengsaa P. Behavioural risk factors for cervical cancer from a prospective study in Khon Kaen, Northeast Thailand. Cancer Detect Prev. 2004. 28:334–339.
22. Misra JS, Gupta HP, Das V. Assessing the feasibility of single lifetime PAP smear evaluation between 41-50 years of age as strategy for cervical cancer control in developing countries from our 32 years of experience of hospital-based routine cytological screening. Diagn Cytopathol. 2004. 31:376–379.
23. Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet. 2004. 364:249–256.
24. Baldwin P, Laskey R, Coleman N. Translational approaches to improving cervical screening. Nat Rev Cancer. 2003. 3:217–226.
25. Schoell WM, Janicek MF, Mirhashemi R. Epidemiology and biology of cervical cancer. Semin Surg Oncol. 1999. 16:203–211.
26. Saslow D, Runowicz CD, Solomon D, Moscicki AB, Smith RA, Eyre HJ, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002. 52:342–362.
27. Force USPST. Guide to Clinical Preventive Services. 1996. 2nd. Alexandria, VA: International Medical Publishing.
28. Coker AL, Rosenberg AJ, McCann MF, Hulka BS. Active and passive cigarette smoke exposure and cervical intraepithelial neoplasia. Cancer Epidemiol Biomarkers Prev. 1992. 1:349–356.
29. Trevathan E, Layde P, Webster LA, Adams JB, Benigno BB, Ory H. Cigarette smoking and dysplasia and carcinoma in situ of the uterine cervix. JAMA. 1983. 250:499–502.
30. Tay SK, Tay KJ. Passive cigarette smoking is a risk factor in cervical neoplasia. Gynecol Oncol. 2004. 93:116–120.
31. Trimble CL, Genkinger JM, Burke AE, Hoffman SC, Helzlsouer KJ, Diener-West M, et al. Active and passive cigarette smoking and the risk of cervical neoplasia. Obstet Gynecol. 2005. 105:174–181.
32. Slattery ML, Robison LM, Schuman KL, French TK, Abbott TM, Overall JC Jr, et al. Cigarette smoking and exposure to passive smoke are risk factors for cervical cancer. JAMA. 1989. 261:1593–1598.
33. Prokopczyk B, Cox JE, Hoffmann D, Waggoner SE. Identification of tobacco-specific carcinogen in the cervical mucus of smokers and nonsmokers. J Natl Cancer Inst. 1997. 89:868–873.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download