Abstract
A 37-year-old woman was admitted to Dong-A University Hospital for rapidly progressive congestive heart failure. Transthoracic echocardiography demonstrated a large mass with a stalk that appeared to be a myxoma on the posterior wall of the left atrium. However, the histological diagnosis was undifferentiated pleomorphic sarcoma. We report a case of primary undifferentiated pleomorphic sarcoma of the left atrium with acute pulmonary edema caused by mitral inflow obstruction.
Primary neoplasms of the heart are rare. Autopsy series report a combined incidence of 0.0017% to 0.28% for both benign and malignant primary cardiac tumors.1,2 Malignant tumors, the majority of which are sarcomas, comprise up to 25% of cardiac neoplasms.3 Undifferentiated cardiac sarcomas primarily develop on the left side of the heart and cause signs and symptoms related to pulmonary congestion, mitral stenosis, and pulmonary vein obstruction.4
We present a case of cardiac undifferentiated pleomorphic sarcoma that presented as acute pulmonary edema, and was preoperatively diagnosed as a benign myxoma of the left atrium.
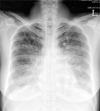
A 37-year-old woman was admitted to our hospital with rapidly progressive dyspnea for 3 days. On the day of admission, she was in severe respiratory distress with orthopnea. She had been diagnosed with hypertension 3 years earlier, which has been well controlled with medication. Her blood pressure was 110/70 mmHg; pulse, 135 beats/min; and respirations, 24 breaths/min. Cardiac auscultation revealed a grade III/VI diastolic murmur at the apex. The electrocardiogram showed sinus tachycardia. Chest radiography revealed bilateral pulmonary edema (Fig. 1). Laboratory studies were normal. A two-dimensional echocardiogram disclosed a large mass with a stalk on the posterior wall of the left atrium, measuring 4.5 × 3.4 cm (Fig. 2). The mass did not prolapse into the left ventricle, but extended into the mitral annulus and obstructed transmitral inflow. Color Doppler demonstrated flow turbulence across the mitral valve. Continuous-wave Doppler showed a severe mitral stenosis with a maximal velocity of 3.1 m/s and a mean pressure gradient of 20 mmHg through the mitral valve (Fig. 3).
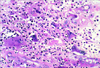
The operation was performed through a median sternotomy with a mild hypothermic cardiopulmonary bypass. Intraoperatively, two separate tumors were identified (Fig. 4). The main mass, observed on echocardiogram, originated from the posterior wall of the left atrium and extended into the pulmonary veins. The mass was 4 × 3.5 × 3.5 cm in size and dark red in color. The mass was excised and the posterior wall of left atrium was repaired. The second yellow mass, which was not detected by the echocardiogram, was attached to the interatrial septum and extended to the atrial aspect of the posterior mitral leaflet; it measured 5.5 × 3 × 1.5 cm in size. The tumor was almost completely excised, except for the small portion of mass on the posterior mitral leaflet. The defect of the interatrial septum secondary to the operation was repaired. We did not repair or replace the mitral leaflet. The macroscopic appearance of the main mass (Fig. 4, right side) was semi-solid and ball-shaped. The mass had a smooth outer surface and showed a homogenous appearance with a focal cystic change filled with a blood clot. The second mass (Fig. 4, left side) had a pinkish-yellow myxoid appearance with hemorrhagic spots and blood clots. Histologically, the tumor was composed of spindled or epithelioid pleomorphic cells with oval nuclei, prominent nucleoli, and abundant eosinophilic cytoplasm. In addition, intermixed giant cells were common. The neoplastic cells exhibited a high degree of nuclear pleomorphism and mitotic activity (Fig. 5). Focal necrosis was also present. On immunohistochemical staining, neoplastic cells showed positive immunoreactivity for vimentin and desmin but were negative for S-100 protein, CD34, CD31, smooth muscle actin, and cytokeratin. The histologic diagnosis was undifferentiated pleomorphic sarcoma. Postoperative metastatic work-up showed no evidence of metastasis. The patient did not receive chemotherapy or radiation and is still alive at present, 6 months after the operation
Primary malignant tumors of the heart are extremely uncommon,1,2 and almost all are sarcomas. There are several types of sarcomas, including angiosarcoma, rhadomyosarcoma, fibrous histiocytoma, spindle cell sarcoma, fibrosarcoma, synovial sarcoma, myxosarcoma, and unclassified sarcoma.5 Malignant fibrous histiocytoma is now regarded as synonymous with undifferentiated sarcoma.4 Undifferentiated pleomorphic sarcoma is a high-grade malignancy showing fibroblastic or myoblastic differentiation and areas of marked cellular pleomorphism. All primary tumors of the heart, whether malignant or benign, are potentially lethal because of intracavitary or valvular obstruction, peripheral embolization, and/or rhythm disturbances. Therefore, surgery should be performed as soon as possible after a cardiac tumor is found.6
Surgical resection of benign myxoma results in full recovery in most cases, whereas resection of malignant sarcoma leads to only palliation of the symptoms and does not contribute to long-term survival.7 Undifferentiated pleomorphic sarcomas proliferate rapidly, and generally patients die of progressive heart failure, although distant metastases are also encountered at diagnosis.8 The key diagnostic tool for cardiac tumors is transthoracic and transesophageal echocardiography. Typically, this diagnostic test accurately identifies the shape, size, possible valve involvement, and myocardial invasion of the tumor, thus providing information useful for determining the optimal therapeutic approach. However, it is difficult to determine the specific type of tumor from echocardiographic findings.
Previous studies have demonstrated that two types of consistency are detectable, even in benign myxomas, by two-dimensional echocardiography. Of 25 cases with atrial myxoma, 18 had a deformable and jelly-like echocardiographic appearance, whereas the others were non-deformable and firm.9 In the present case, the preoperative echocardiographic diagnosis was a benign myxoma, but histological and immunohistochemical studies revealed a malignant, undifferentiated pleomorphic sarcoma. Malignant fibrous histiocytoma tends to be located in the left atrium of the heart, most commonly on the posterior wall and/or interatrial septum.10-12 In a recent review, 38 (81%) of 47 cases were found in the left atrium.13 The majority of malignant fibrous histiocytomas develop in the left atrium and they more commonly arise along the posterior wall in comparison to the septum.10-12 Benign myxoma is usually a solitary tumor;6,14,15 however, undifferentiated cardiac sarcoma may form multiple masses.12,16-18
In conclusion, because the majority of cardiac undifferentiated pleomorphic sarcomas occur in the left atrium, these growths can be mistakenly diagnosed as benign myxoma preoperatively. However, careful echocardiographic preoperative evaluation, including tumor origin and multiple growth profile, may be helpful in identifying malignant cardiac tumors.
Figures and Tables
Fig. 2
Transthoracic echocardiogram in apical 4 chamber view. A large mobile mass attached on posterior wall of left atrium was observed.
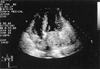
Fig. 3
Doppler echocardiogram in apical 4 chamber view. Continuous-wave Doppler showed a severe mitral stenosis with a maximal velocity of 3.1 m/s and a mean pressure gradient of 20 mmHg through the mitral valve.
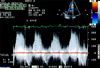
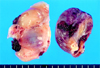
Fig. 4
Photographs of the resected tumors. The main mass (right side) observed in echocardiogram was resected from the posterior wall of the left atrium, measuring 4 × 3.5 × 3.5 cm. The second mass (left side) was not detected in echocardiogram and was found at the interatrial septum and posterior mitral leaflet, measuring 5.5 × 3 × 1.5 cm.
References
1. Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996. 77:107.
2. Lam KY, Dickens P, Chan AC. Tumors of the heart: a 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med. 1993. 117:1027–1031.
3. Silverman NA. Primary cardiac tumors. Ann Surg. 1980. 191:127–138.
4. Burke AP, Tazelaar H, Butany JW, El-Demellawy D, Loire R, Geva T, et al. Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, editors. Cardiac sarcomas. Pathology and genetics of tumours of the lung, pleura, thymus and heart. 2004. Lyon: IARC Press;276–277.
5. Meng Q, Lai H, Lima J, Tong W, Qian Y, Lai S. Echocardiographic and pathologic characteristics of primary cardiac tumors: a study of 149 cases. Int J Cardiol. 2002. 84:69–75.
6. Shapiro LM. Cardiac tumors: diagnosis and management. Heart. 2001. 85:218–222.
7. Miralles A, Bracamonte L, Soncul H, Diaz del Castillo R, Akhtar R, Bors V, et al. Cardiac tumors: clinical experience and surgical results in 74 patients. Ann Thorac Surg. 1991. 52:886–895.
8. Allard MF, Taylor GP, Wilson JE, McManus BM. Goldhaber SZ, Braunwald E, editors. Primary cardiac tumors. Atlas of heart disease. 1995. vol 3: cardiopulmonary disease and cardiac tumors. St Louis: Mosby-Year Book;1–22.
9. Fyke FE 3rd, Seqard JB, Edwards WD, Miller FA Jr, Reeder GS, Schattenberg TT, et al. Primary cardiac tumors: experience with 30 consecutive patients since the introduction of two-dimensional echocardiography. J Am Coll Cardiol. 1985. 5:1465–1473.
10. Korbmacher B, Doering C, Schulte HD, Hort W. Malignant fibrous histiocytoma of the heart: case report of a rare left atrial tumor. Thorac Cardiovasc Surg. 1992. 40:303–307.
11. Laya MB, Maillliard JA, Bewtra C, Levin HS. Malignant fibrous histiocytoma of the heart; a case report and review of the literature. Cancer. 1987. 59:1026–1031.
12. Ovcak Z, Masera A, Lamovec J. Malignant fibrous histiocytoma of the heart. Arch Pathol Lab Med. 1992. 116:872–874.
13. Okamoto K, Kato S, Katsuki S, Wada Y, Toyozumi Y, Morimatsu M, et al. Malignant fibrous histiocytoma of the heart: case report and review of 46 cases in the literature. Intern Med. 2001. 40:1222–1226.
14. Endo A, Ohtahara A, Kinugawa T, Mori M, Fujimoto Y, Yoshida A, et al. Characteristics of 161 patients with cardiac tumors diagnosed during 1993 and 1994 in Japan. Am J Cardiol. 1997. 79:1708–1711.
15. Kang WC, Ha JW, Chang BC, Kwon JW, Rim SJ, Chung NS, et al. A review of cardiac myxoma: 33-year experience in a single institution. Korean Cir J. 1998. 28:1131–1140.
16. Orlandi A, Ferlosio A, Dell'Anna V, Quitadamo R, Pellegrino A, Spagnoli LG. Undifferentiated sarcoma of the heart: a rare clinicopathologic presentation. J Thorac Cardiovasc Surg. 2002. 124:192–193.
17. Hasegawa T, Nakagawa S, Chino M, Kunihiro T, Ui S, Kimura M. Primary cardiac sarcoma mimicking benign myxoma: a case report. J Cardiol. 2002. 39:321–325.
18. Kim JT, Baek WK, Kim KH, Yoon YH, Kim DH, Lim HK. A primary cardiac sarcoma preoperatively presented as a benign left atrial myxoma. Yonsei Med J. 2003. 44:530–533.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download