Abstract
We report a 60-year-old woman with intramucosal adenocarcinoma arising in the interposed colon, 40 years after the esophageal reconstruction for lye induced esophageal stricture. Although synchronous adenomas were also found in the native colon where the graft was taken, the number of adenomas was greater in the interposed colon and more dysplastic, even progressed to adenocarcinoma, than that of the native colon. The microsatellite instability-testing performed in the intramucosal carcinoma from interposed colon showed absence of microsatellite instability. Changing of location and functional deman]d of colonic segment, and the exposure to different intraluminal contents might have facilitated the adenomacarcinoma transformation in the interposed colon.
Colon graft has been a favored surgical technique for benign esophageal stricture and esophageal malignancy. Early post-operative complications of this procedure are common and include anastomosis leaks, fistula formation, colon graft edema, ischemia, and stricture.1-4 Late complications include peptic ulcer, fistula formation, reflux colitis, atonia, outlet obstruction in stomach, and rarely malignancy. We report a rare case of colonic adenoma with carcinomatous transformation arising in the interposed colon.
A 60-year-old woman, who had undergone esophagectomy with colon interposition, due to esophageal lye-stricture 40 years ago, was admitted to hospital with hematemesis and heartburn sensation. Laboratory evaluation including hematological and biochemistry studies were unremarkable except for mild anemia. Endoscopic examination of the colonic graft could not find the focus of the bleeding but six polyps of 3 to 5 mm in size were found incidentally. Colonoscopy of the native colon revealed two polyps of similar size, one in the ascending colon, and the other in the cecum. Consequently, endoscopic and colonoscopic polypectomy was carried out. Histologically, all resected specimens were adenomatous polyps and only two polyps from interposed colon showed high grade dysplasia, and others showed no dysplasia.
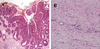
8 months later, the patient was admitted due to melena. Endoscopic examination of the colon graft revealed a 1.0 cm sized, active staged ulceration at cologastric anastomosis. In addition, two flat to slightly elevated lesions of 12 and 20 mm in size which might have been missed at the previous examination, were noted at the anastomosis site and 4 cm proximal to the anastomosis site (Fig. 1 A, B). As there was no evidence of active bleeding, endosopic biopsies were performed in ulcer margin while endoscopic mucosal resection of colonic polyps were carried out (Fig. 1C, D). Pathology of the resected 20 mm sized adenoma disclosed tubulo-villous adenoma with high grade dysplasia and focus of intramucosal well-differentiated adenocarcinoma (Fig. 2A, B). We performed the test for microsatellite instability (MSI)-testing of the lesion. The DNA from the tumor was amplified by polymerase-chain-reaction with the microsatellite markers and genotyped after fluorescence labeling, and the result of MSI-testing was stable (Fig. 3). The patient discharged without further bleeding episode and was treated with H2 receptor antagonist.
Colonic interposition for esophageal reconstruction was described first in 1911. This operation has been generally performed for malignant disease of esophagus and cardia, but also has been largely applied to the benign esophageal disease, such as lye-induced strictures. Because the patients who underwent colonic interposition for benign disease may have normal life-expectancy, late morbidity associated with this operation is particularly important. Malignancy arising in interposed colon is a rare complication of colonic interposition.
In general, the etiology of colon cancer is multifactorial comprising environmental factors, especially diet, and genetic factors. The diet with high animal fat and low fiber has been reported to be associated with carcinomatous transformation of colon.5 The currently accepted hypothesis regarding animal fat is that the ingestion of animal fat leads to an increased proportion of anaerobes in the gut microflora, resulting in the conversion of normal bile acids into carcinogen. The theory related to dietary fiber is that the fiber accelerates intestinal transit time, reducing the exposure time of colonic mucosa to potential carcinogen and diluting these carcinogens. These studies have been performed in the view of indirect effect of diet.
However, interposed colonic segment would have a marked change of location and functional demand. Such changes include direct exposure of colonic mucosa to environmental substances with potential toxicity or carcinogenicity to colonic mucosa, and to different microflora. The colonic mucosa encounters these factors right after the change of location, and also affected by gastric juice and other physiologic factors involved in alimentary tract function. Altorjay et al.6 reported that the muscle contraction of interposed colon was segmental without spreading waves on manometric examination. The passage time of food contents in grafted colon is longer than in normal esophagus, and the grafted colon may well be more vulnerable to the refluxates from stomach due to absence of sphincter. Therefore, the duration of exposure to noxious agent and carcinogen is longer in the grafted colon than the normal esophagus.7 Interestingly, Lindahl et al.8 reported that interposed colonic mucosa tended to undergo frequent premalignant transformation such as fundic type gastric metaplasia of interinterposed colon, colonic dysplasia, and increased aneuploid cell population, in a study with histologic and flow cytometric examination of interposed colonic mucosa.
To date, 10 cases6,9-17 of primary adenocarcinoma arising from interposed colon have been reported worldwide to our knowledge, including the report of Fritscher-Ravens et al.12 in which the tumor in the interposed colon was diagnosed 7 months after esophagectomy, probably developing from undiagnosed polyps in the transposed colon (Table 1). In the current case, synchronous adenomas were found in the native colon, as well. However, number of adenomas found in the interposed colon was larger than that of the native colon, and carcinomatous transformation was found only in the interposed colon. Of course, the left colon, which is mostly used to replace the esophagus is a common site of cancer over the lifespan. Even in the unoperated patient the finding of dysplasia and cancer is more common in the left colon than the right colon. Adenocarcinoma in the interposed colon may only be the result of the natural history of the dysplasiacarcinoma sequence outside the native anatomical location. However, we confirmed that she had undergone transverse colectomy on colonoscopic examination.
Microsatellite instability is a well-recognized feature of non-sporadic colorectal cancer, especially in the hereditary non-polyposis colorectal cancer. However, about 10% of sporadic colorectal cancers have microsatellite instability.18,19 We were concerned about the result of microsatellite instability in the sporadic colorectal tumors under mechanical stress and carcinogens. Our MSI-testing showed the absence of instability further emphasizing the importance of environmental exposures in this case.
We anticipate the average span of patient's life with interposed colon would be long enough in whom esophageal colon graft has been performed for benign diseases of esophagus and stomach. The early and regular screening of the interposed colonic graft to detect and treat malignancy may be demanded because malignancy arising from interposed colon is asymptomatic, but fatal, and re-operation is difficult.
Figures and Tables
Fig. 1
(A, B, C, D) Arrows indicate the flat elevated mucosal lesion in the interposed colon, located at the anastomosis site and measuring 12 mm (A). Another flat elevated lesion located 4 cm proximal to the anastomosis site, measuring 20 mm is indicated with arrow heads (B). These polyps were removed by snare polypectomy after the submucosal injection of saline (C, D).

Fig. 2
(A, B) Pathology of the resected 20 mm sized adenoma showing tubulo-villous adenoma with high grade dysplasia and focus of intramucosal well-differentiated adenocarcinoma. (A × 100 and B × 400).
References
1. Agha FP, Orringer MB. Colonic interposition: radiographic evaluation. AJR Am J Roentgenol. 1984. 142:703–708.
2. Larson TC 3rd, Shuman LS, Libshitz HI, McMurtrey MJ. Complications of colonic interposition. Cancer. 1985. 56:681–690.
3. Lundell L, Olbe L. Colonic interposition for reconstruction after resection of cancer in the esophagus and gastroesophageal junction. Eur J Surg. 1991. 157:189–192.
4. Briel JW, Tamhankar AP, Hagen JA, DeMeester SR, Johansson J, Choustoulakis E, et al. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis:gastric pull-up versus colon interposition. J Am Coll Surg. 2004. 198:536–542.
5. Neagoe A, Molnar AM, Acalovschi M, Seicean A, Serban A. Risk factors for colorectal cancer: an epidemiologic descriptive study of a series of 333 patients. Rom J Gastroenterol. 2004. 13:187–193.
6. Altorjay A, Kiss J, Vörös A, Szanto I, Bohak A. Malignant tumor developed in colon-esophagus. Hepatogastroenterology. 1995. 42:797–799.
7. Bucknell A, Clark C. An experimental method for recording the behaviour of human isolated colonic segments. Gut. 1967. 8:569–573.
8. Lindahl H, Rintala R, Sariola H, Louhimo I. Long-term endoscopic and flow cytometric follow-up of colon interposition. J Pediatr Surg. 1992. 27:859–861.
9. Goldsmith HS, Beattie EJ Jr. Malignant villous tumor in a colon bypass. Ann Surg. 1968. 167:98–100.
10. Licata AA, Fecanin P, Glowitz R. Metastatic adenocarcinoma from oesophageal colonic interposition. Lancet. 1978. 1:285.
11. Haerr RW, Higgins EM, Seymore CH, el-Mahdi AM. Adenocarcinoma arising in a colonic interposition following resection of squamous cell esophageal cancer. Cancer. 1987. 60:2304–2307.
12. Houghton AD, Jourdan M, McColl I. Dukes A carcinoma after colonic interposition for oesophageal stricture. Gut. 1989. 30:880–881.
13. Theile DE, Smithers M, Strong RW, Windsor CJ. Primary adenocarcinoma in a colonic "oesophageal" segment. Aust N Z J Surg. 1992. 62:158–160.
14. Lee SJ, Koay CB, Thompson H, Nicolaides AR, Das Gupta AR. Adenocarcinoma arising in an oesophageal colonic interposition graft. J Laryngol Otol. 1994. 108:80–83.
15. Fritscher-Ravens A, Sriram PV, Thonke F, Jaeckle S, Maydeo A, Soehendra N. Synchronous adenocarcinoma in the transposed colonic conduit after esophagectomy for squamous cell cancer: endoscopic palliative resection while awaiting surgery. Gastrointest Endosc. 1999. 50:852–854.
16. Goyal M, Bang DH, Cohen LE. Adenocarcinoma arising in interposed colon: report of a case. Dis Colon Rectum. 2000. 43:555–558.
17. Liau CT, Hsueh S, Yeow KM. Primary adenocarcinoma arising in esophageal colon interposition: report of a case. Hepatogastroenterology. 2004. 51:748–749.
18. Jass JR. HNPCC and sporadic MSI-H colorectal cancer: a review of the morphological similarities and differences. Fam Cancer. 2004. 3:93–100.
19. de la Chapelle A. Microsatellite instability. N Engl J Med. 2003. 349:209–210.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download