Abstract
Patients with primary small cell carcinoma of the liver have rarely been described in medical literature. Knowledge of clinical, pathological and immunohistochemical properties remains limited. We described an 82-year-old female patient with primary small cell carcinoma of the liver. Histologically, the tumor showed typical morphology of a pulmonary small cell carcinoma. Immunohistochemically, the tumor revealed neuroendocrine differentiation; positive reaction for chromogranin, synaptophysin, CD56, and neuron specific enolase. The tumor was also positive for TTF-1 and c-kit but completely negative for hepatocyte, carcinoembryonic antigen, cytokeratin 7; 19; and 20. Herein, we discussed the clinical, pathological and immunohistochemical findings of extrapulmonary small cell carcinoma of the liver and reviewed the relevant literature.
The most common site of small cell carcinoma is the lung. It has rarely been found at extrapulmonary sites such as the trachea, larynx, thymus, esophagus, stomach, small intestine, colon, prostate, gallbladder, skin, breast, and uterine cervix.1 Small cell carcinoma, involving primarily the liver, is extremely rare; and only nine cases have been reported in the literature.2-7 The clinical and pathological features as well as immunohistochemical findings have rarely been reported, furthermore the reported findings are not always consistent. Here, we report a case of extrapulmonary small cell carcinoma of the liver and review of the medical literature.
An 82-year-old female with hypertension complained of abdominal discomfort in the right upper quadrant. She had undergone cholecystectomy and T-tube choledochostomy 2 years earlier because of gallbladder and common bile duct stones. Abdominal ultrasonography and computed tomography revealed a 5.6 cm-sized liver mass with peripheral rim enhancement (Fig. 1). Lymph node enlargement was present at the aorto-caval area. The patient did not smoke and was not an alcoholic. Colonoscopy showed a tubular adenoma at the sigmoid colon. All laboratory tests, including liver function testing, peripheral blood counts, and tumor markers such as carcinoembryonic antigen(CEA), CA19 - 9 and alpha fetoprotein, were in the normal range. HBsAg was negative and HBsAb was positive. Anti-HCV and anti-HIV were negative.
Surgical resection of segment 6 of the liver and right hemicolectomy due to hepatic adhesion was performed. The tumor and non-tumor liver tissue were formalin-fixed and paraffin embedded. After surgery, bronchial washing, chest computed tomography (CT) and PET-CT were performed to exclude primary pulmonary small cell carcinoma; and there was no evidence of lung cancer. Post operative chemotherapy was not performed because the patient refused further treatment due to her advanced age. Seven months after surgery, 1.1 cm to 2.8 cm-sized multiple hepatic nodules developed with enlargement of the lymph nodes at the porta hepatic, aortocaval, and portocaval areas. No pulmonary abnormalities were detected. Oral etoposide treatment was started because the patient's general condition was poor and she had been receiving anticoagulant therapy due to atrial fibrillation. Two months after chemotherapy, the size of the hepatic nodules and lymph nodes decreased. The patient is currently alive 1.5 years post-surgery without significant problems.
Paraffin blocks were used for hematoxylin-eosin staining and immunohistochemistry. The primary antibodies are listed in Table 1.
Grossly, the tumor was 6.7 × 5.5 × 5.5 cm with a nodular expanding tumor border (Fig. 2) that involved Glisson's capsule and invaded the pericolic fat. The cut surface of the tumor was yellow, tan, and friable with necrotic areas. Portal vein invasion was absent. The background liver was not cirrhotic.
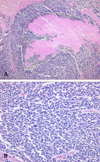
Histologically, the tumor was composed of small round cells with multifocal necrosis, morphologically similar to pulmonary small cell carcinoma (Fig. 3A). An insular and trabecular pattern was not observed. The tumor cells showed hyperchromatic nuclei with a "salt and pepper" pattern of finely dispersed chromatin, indistinct nucleoli, and frequent mitoses (Fig. 3B). Nuclear moldings and crush artifacts were present. The tumor cells were diffusely positive for synaptophysin, chromogranin, CD56, neuron specific enolase (NSE), thyroid transcription factor-1 (TTF-1) and c-kit (Fig. 4A, B). On the other hand, cytokeratin 7; 19; and 20; CEA; alpha fetoprotein; hepatocyte; vimentin; desmin; and S-100 protein were all negative. There was no hepatocellular carcinoma or adenocarcinoma component. Multiple enlarged lymph nodes were identified two of which showed metastatic small cell carcinoma.
Extrapulmonary small cell carcinoma has been reported to occur in 0.1 - 0.4% of all malignancies.8 Extrapulmonary small cell carcinoma shows neuroendocrine differentiation, but it is not synonymous with neuroendocrine carcinoma.
Primary neuroendocrine carcinomas of the liver may show solid, trabecular and insular arrangements reminiscent of a carcinoid;9-12 however, extrapulmonary small cell carcinoma of the liver is histologically indistinguishable from metastatic pulmonary small cell carcinoma to the liver. Therefore, exclusion of pulmonary small cell carcinoma is a prerequisite for the diagnosis of extrapulmonary small cell carcinoma. In our case, the tumor showed homogeneous small cell features without a carcinoid-like pattern. There was no pulmonary lesion detected on a chest X-ray, chest CT, PET-CT, or bronchoscopy. Approximately half of the small cell carcinomas that develop in the gastrointestinal tract contain non-small cell carcinoma elements;13 but our case had pure small-cell morphology without features of hepatocellular carcinoma or biliary adenocarcinoma.
The results of immunohistochemical staining of small cell carcinoma occurring in the liver vary. Recently reported cases, including this case, were positive for more than 2 neuroendocrine markers such as CD56, synaptophysin, or chromogranin.2,3,8 Although TTF-1 is usually positive in pulmonary small cell carcinomas (about 96%),14 its expression is not constant in extrapulmonary small cell carcinoma. Two cases of small cell carcinoma of the liver previously reported were negative for TTF-1,2,3 but our case was positive. TTF-1 expression has been reported in some extrapulmonary small cell carcinomas occurring in the gastrointestinal tract, urinary bladder, uterine cervix, prostate, thyroid gland, and breast and it is not a specific marker for pulmonary small cell carcinoma.14-17 Cytokeratin expressions have been studied in small cell carcinomas of the liver.3,5 Kim et al.3 reported that a tumor was negative for CK7, 19 and 20, in agreement with our case. However, Zanconati et al.5 reported that AE1/AE3, cytokeratin 8, 18, and 19 were positive for tumors in all of the 3 cases that they reported. These cytokeratins are expressed more commonly in cholangiocarcinoma than in hepatocellular carcinoma and have negative reactions with neuroendocrine markers except for NSE, which is less specific than chromogranin. The immunophenotype of the 3 cases reported by Zanconati et al.5 were more compatible with a carcinoma showing biliary differentiation rather than neuroendocrine differentiation. The study of more cases is needed to characterize in detail the immunophenotype of primary hepatic small cell carcinoma.
Regarding the origin of neuroendocrine cells, differentiation and proliferation from biliary epithelium have been favored because bile ducts contain neuroendocrine argentaffin cells. However, neuroendocrine differentiation from stem cell theory should also be considered because the tumor cells were found to express c-kit, a stem cell marker of the liver, in 4 recently reported cases including ours.
The clinical and pathological findings of primary small cell carcinoma of the liver are summarized in Table 2. The clinical features such as low alpha fetoprotein and absence of viremia or cirrhosis are different from conventional hepatocellular carcinoma. Extrapulmonary small cell carcinomas generally show rapid progression with distant metastases and poor prognosis. Small cell carcinoma of the liver is rare and the information on the clinical course is limited. Sengoz et al.4 reported 2 cases of extrapulmonary small cell carcinoma of the liver, 1 of which survived for 13 months after chemotherapy and the other for 67 months after right hemihepatectomy. Zanconati et al.5 reported 2 cases that were acutely fatal. In our case, the patient did not receive the standard chemotherapy of small cell carcinoma because of her advanced age, poor general condition, and atrial fibrillation. Fortunately, she showed response to oral etoposide that was given after recurrence. She is still alive after 1.5 years later without significant clinical complications. In general, the treatment of extrapulmonary small cell carcinoma is organ resection and adjuvant chemotherapy using the same regimen as with pulmonary small cell carcinomas.
Figures and Tables
Fig. 3
Microscopic findings of the tumor reveal solid small round cells and necrosis (A). The tumor cells show oval to fusiform hyperchromatic nuclei and indistinct nucleoli with frequent mitoses (B).
References
1. Richardson RL, Weiland LH. Undifferentiated small cell carcinomas in extrapulmonary sites. Semin Oncol. 1982. 8:484–496.
2. Ryu SH, Han SY, Suh SH, Koo YH, Cho JH, Han SH, et al. A case of primary small cell carcinoma of the liver. Korean J Hepatol. 2005. 11:289–292.
3. Kim YH, Kwon R, Jung GJ, Roh MH, Han SY, Kwon HC, et al. Extrapulmonary small-cell carcinoma of the liver. J Hepatobiliary Pancreat Surg. 2004. 11:333–337.
4. Sengoz M, Abacioglu U, Salepci T, Eren F, Yumuk F, Turhal S. Extrapulmonary small cell carcinoma: multimodality treatment results. Tumori. 2003. 89:274–277.
5. Zanconati F, Falconieri G, Lamovec J, Zidar A. Small cell carcinoma of the liver: a hitherto unreported variant of hepatocellular carcinoma. Histopathology. 1996. 29:449–453.
6. Kim KJ, Yim HJ, Kim MJ, Choung RS, Yeon JE, Lee HS, et al. A case of primary small cell neuroendocrine carcinoma of the liver. Korean J Gastroenterol. 2006. 48:37–41.
7. Kim KO, Lee HY, Chun SH, Shin SJ, Kim MK, Lee KH, et al. Clinical overview of extrapulmonary small cell carcinoma. J Korean Med Sci. 2006. 21:833–837.
8. Vrouvas J, Ash DV. Extrapulmonary small cell cancer. Clin Oncol (R Coll Radiol). 1995. 7:377–381.
9. Dala R, Shoosmith J, Lilenbaum R, Cabello-Inchausti B. Primary hepatic neuroendocrine carcinoma: an underdiagnosed entity. Ann Diagn Pathol. 2006. 10:28–31.
10. Hsueh C, Tan XD, Gonzalez-Crussi F. Primary hepatic neuroendocrine carcinoma in a child. Morphologic, immunocytochemical, and molecular biologic studies. Cancer. 1993. 71:2660–2665.
11. Pilichowska M, Kimura N, Ouchi A, Lin H, Mizuno Y, Nagura H. Primary hepatic carcinoid and neuroendocrine carcinoma: clinicopathological and immunohistochemical study of five cases. Pathol Int. 1999. 49:318–324.
12. Kaya G, Pasche C, Osterheld MC, Chaubert P, Fontolliet C. Primary neuroendocrine carcinoma of the liver: an autopsy case. Pathol Int. 2001. 51:874–878.
13. Brenner B, Tang LH, Klimstra DS, Kelsen DP. Small-cell carcinomas of the gastrointestinal tract: a review. J Clin Oncol. 2004. 22:2730–2739.
14. Ordonez NG. Value of thyroid transcription factor-1 immunostaining in distinguishing small cell lung carcinomas from other small cell carcinomas. Am J Surg Pathol. 2000. 24:1217–1223.
15. Kaufmann O, Dietel M. Expression of thyroid transcription factor-1 in pulmonary and extrapulmonary small cell carcinomas and other neuroendocrine carcinomas of various primary sites. Histopathology. 2000. 36:415–420.
16. Jones TD, Kernek KM, Yang XJ, Lopez-Beltran A, MacLennan GT, Eble JN, et al. Thyroid transcription factor 1 expression in small cell carcinoma of the urinary bladder: an immunohistochemical profile of 44 cases. Hum Pathol. 2005. 36:718–723.
17. Yao JL, Madeb R, Bourne P, Lei J, Yang X, Tickoo S, et al. Small cell carcinoma of the prostate: an immunohistochemical study. Am J Surg Pathol. 2006. 30:705–712.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download