INTRODUCTION
Multiple occurrences of primary neoplasms is relatively well known, but simultaneous occurrence of malignant tumors of different histologic types is rare. Specifically, synchronous manifestation of primary adenocarcinoma and MCL within the same stomach has never been reported. MCL is a well-recognized subtype of B-cell lymphoma associated with a very poor prognosis.1-3 The incidence of MCL is approximately 2 - 3/100,000/year, which represents approximately 5 - 10% of all lymphoma cases in North America and Europe.1,4,5 MCL comprises 2.5 - 7% of non-Hodgkin's lymphomas (NHLs) and involves the gastrointestinal tract in up to 30% of cases.4-6 Important findings about the molecular biology and genetics of MCL have recently been made.7,8 However, MCL is still characterized by a poor prognosis due to its aggressive clinical course. The median survival of the disease is only 3 years, and only 10 - 15% of the patients are long-term survivors.1-3,8,9 A satisfactory standard treatment for a cure has not yet been established. Furthermore, little is known about the coexistence of gastric mantle cell lymphoma and gastric adenocarcinoma. We hereby report a rare case of synchronous gastric adenocarcinoma and MCL detected incidentally in the area of the middle third of the stomach.
CASE REPORT
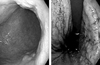
A 62-year-old man presented with an abnormal gastric mucosal lesion (Fig. 1) during a routine check-up for evaluation of generalized weakness. The lesion was diagnosed as gastric cancer by a gastrointestinal endoscopy. He had been previously diagnosed with diabetes mellitus, Alzheimer's disease, and renovascular hypertension. He had no symptoms associated with the gastrointestinal tract and no history of a Helicobacter pylori infection. Superficial lymph nodes were not palpable. All other physical examinations were unremarkable.
A complete blood cell count showed hemoglobin 15.4 g/dL, hematocrit 44.4%, platelet 249,000/µL, and white blood corpuscle 8,500/µL. On a tumor marker study, alpha fetoprotein (AFP) was 1.8 ng/mL, carcinoembryonic antigen (CEA) was 4.9 ng/mL, and carbohydrate antigen (CA) 19 - 9 was 5.5 U/mL. Other laboratory findings were within normal limits. Upon endoscopy, there were two mucosal abnormalities. One abnormality was a relatively well demarcated lesion, 3.5 cm in size, with an irregular margin just above the gastric angle. Another 3 cm, whitish, and elevated lesion with central ulceration was noted on the greater curvature side of a body. Microscopic examination of an endoscopic biopsy specimen of the first lesion revealed a carcinoma, and the latter revealed dysplasia. Simultaneously performed abdominal computed tomography showed slightly enlarged perigastric lymph nodes without distant metastasis. Ultrasonography and a whole body bone scan showed no significant findings.
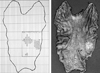
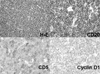
We performed a radical total gastrectomy with a splenectomy and Roux-en-Y esophagojejunostomy. Macroscopically, there were two flat and oval shaped, plaque-like lesions on the lesser and greater curvature of the body. The sizes of each lesion were 3.5 cm by 3.0 cm and 3.0 cm by 2.5 cm, respectively (Fig. 2). Microscopically, the smaller lesion in the greater curvature was tubular adenoma with well differentiated adenocarcinoma confined to gastric mucosa (Fig. 3). There were no lymph node metastases of the carcinoma in any of the 39 lymph nodes. The larger lesion of the lesser curvature showed multiple nodular proliferations of small lymphoid cells mainly in the mucosa with focal submucosal extension (Fig. 4). MCL cells were found in 27 out of the 39 retrieved lymph nodes. Immunohistochemical staining of the small lymphoid cells showed positive reaction for CD5, CD20, and cyclin D1 (Fig. 5) and negative reaction for CD3 and MT1. These findings were compatible with synchronous manifestation of EGC IIc (Stage Ia) and MCL (stage III). The patient made an uneventful postoperative recovery and did not receive any adjuvant chemotherapy due to his refusal.
DISCUSSION
Although many reports have been published about multiple carcinomas of the stomach, few have reported the coexistence of gastric carcinoma and gastric malignant lymphoma.10-12 There has especially been no report about synchronous primary adenocarcinoma and MCL of the stomach. Only a few cases of synchronous adenocarcinoma and MCL of the colon have been reported in western countries.13,14 The incidence of colorectal cancer in western countries has been high, even though the tumorigensis of adenocarcinoma and MCL are different, complex, and multifactorial. Interestingly, the present synchronous occurrence of gastric adenocarcinoma and MCL in the same stomach seems to be by chance because the incidence of gastric adenocarcinoma in Korea is higher than that in western countries.
MCL was individualized years ago under several different names. The term MCL was proposed for unification of morphologic, immunologic, and molecular data.15 The term MCL represents a subtype of lymphomas previously classified as centrocytic lymphoma by Kiel classification,16 intermediate cell lymphoma by Berard et al.,17 and diffuse small-cleaved cell lymphoma in the Working Formulation.18 In contrast to other lymphoma subtypes, the etiology and pathogenesis of MCL are not well known. Interestingly, one report suggested that trisomy 3 might be a common chromosomal abnormality in lymphomatous polyps of the mantle cell type.19 The median age of patients is 54-68 years, and the male to female ratio is 2-3:1 at diagnosis.9,20 Approximately 95% of patients present with an advanced stage (Ann Arbor stage III-IV).5,8,20 In the patient of this report, the age, gender, and stage appear to be similar to patients of previous reports.5,8,9,20
The symptoms and signs of gastrointestinal MCL include abdominal pain, diarrhea, hematochezia, nausea, vomiting, weight loss, and fatigue. Gastrointestinal involvement may occur in 10 - 30% of patients either at presentation or during the course of the disease.4,6,9 Upper gastrointestinal involvement was more common than involvement of the colon.9 A study of endoscopic findings of the upper and lower gastrointestinal tract are as follows (exclusive of normal finding): inflammation (29.2%, 0%); nodules and polyps (50%, 92.6%); ulcer (12.5%, 3.7%); thickened wall (4.2%, 3.7%); and mass (4.2%, 0%).6 Extra-nodal involvement is found in approximately 90% of cases; hence, diagnoses at advanced Ann Arbor stages III or IV are frequent.5,9,10 B-symptoms are present in less than 50% of the cases.2,8
Diagnosis of MCL is able to be more accurately made due to the advancement in technology of immunology and molecular genetics. The International Lymphoma Study Group (ILSG) published 'Revised European American Classification of Lymphoid Neoplasm' integrating all the morphologic, immunologic, genetic, and molecular biologic information. Thus, MCL was classified as one of the independent subtypes of 11 peripheral B-cell lymphomas.21 Differential diagnosis from other lymphomas by morphologic findings alone is difficult;22 therefore, immunohistochemical staining is necessary for diagnosis. MCL is typically positive for CD5, CD19, CD20, CD22, CD79a, and cyclin D1. MCL is usually negative for CD10 and CD23.1,8,23,24 CD5 is particularly helpful in differential diagnosis with follicular lymphoma. CD5 is positive for MCL and small lymphocytic lymphoma. CD5 is negative for follicular lymphoma.1,8 The most valuable marker for the diagnosis of MCL is cyclin D1. Overexpression of cyclin D1 indicates bcl-1 gene rearrangement, which is due to the occurrence of t (11;14) in 70% of cases.23,24 In our case, immunohistochemical staining showed positive results for CD5, CD20, and cyclin D1 and negative results for CD3 and MT1. In patients with coexistent lymphoma and carcinoma, two neoplasms are usually located separately. The lymphoma is usually advanced, and carcinoma is well-differentiated.12 Interestingly, in our case the lesions were in the same situation as those in the cited reports. Because MCL has the poorest long-term survival, a wait- and-see strategy is not an appropriate approach. Different chemotherapeutic regimens achieve overall survival of 52 months with complete remission in 20 - 40% of patients.2,5,25 Therefore, we planned to use adjuvant chemotherapy focused on the MCL because there was no necessity for chemotherapy in EGC. Unfortunately, the presented patient refused to receive any adjuvant chemotherapy due to personal reasons.
Important clinical prognostic factors of MCL that have been revealed in previous studies are poor performance status, advanced clinical stage, splenomegaly, anemia, infiltration of bone marrow and peripheral blood, transformation to the blastic variant, mitotic index > 2.5, p53 mutations, elevation of lactate dehydrogenase (LDH) level, and age.20 However, the prognosis of patients with synchronous gastric adenocarcinoma and MCL has yet to be clarified due to the lack of any long-term follow-up observation.
Synchronous development of these two tumors is one of the most challenging problems in diagnosis and treatment. Since there are no typical endoscopic findings or gross findings of gastric adenocarcinoma different from other lymphomas, synchronous carcinomas may be misinterpreted as multifocal separated lesions of lymphoma because of their macroscopic resemblance. Therefore, gastric lymphoma patients should be carefully examined by endoscopy. Suspicious areas must be biopsied considering the possibility of coexisting adenocarcinomas. Moreover, considerable improvements have been accomplished in the understanding and therapeutic approaches of the disease. However, the treatment outcome is still highly unsatisfactory. Therefore, new therapeutic strategies and follow up studies are needed to improve the clinical outcome of synchronous gastric adenocarcinoma and MCL.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download