INTRODUCTION
Radiofrequency (RF) catheter ablation of left free-wall accessory pathways (APs) is performed with the retrograde transaortic or transseptal approach depending on the operator's preference with an equal success rate.1 Transradial coronary angiography and intervention are preferred because of the early ambulation and avoidance of complications related to the femoral arterial puncture.2-6 In contrast to the widespread use of the radial artery in coronary intervention, there are few reports on its use in RF catheter ablation. Here we report a case of an overt left free- wall bypass tract that was successfully ablated via a radial artery.
CASE REPORT
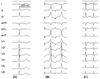
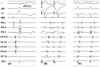
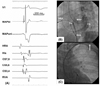
A 37-year-old man with Wolff-Parkinson-White syndrome was referred for an electrophysiologic study (EPS) and ablation. The patient had been suffering from recurrent episodes of palpitations for 2 years. The 12-lead ECG during sinus rhythm exhibited delta waves with a negative polarity in leads I and aVL and positive polarity in lead V1, indicating a left free-wall AP (Fig. 1A). The physical examination, chest X-ray, 2-D and Doppler echocardiography were normal. He had neither claudications nor evidence of peripheral arterial disease upon physical examination. After a written informed consent was obtained from the patient, an EPS was performed with the patient in a fasting state and sedation with midazolam and fentanyl. Three 6-French multipolar electrode catheters were introduced into the right femoral vein and positioned in the high right atrium (HRA), His-bundle recording region (HIS) and the right ventricular apex (RVA). A 6-French decapolar electrode catheter was introduced into the right internal jugular vein and advanced into the coronary sinus (CS). Programmed electrical stimulation was performed with a stimulus of 0.2 ms duration at twice the diastolic threshold. The surface and intracardiac ECGs were simultaneously recorded by way of a dedicated computerized system (CardioLab™, Purucka Engineering Inc., Houston, TX, USA). The local intracardiac electrogram was filtered between 50-500 Hz. The basic intervals were as follows: sinus cycle length = 995 ms, PR interval = 120 ms, QRS duration =140 ms, QT interval = 400 ms, PA interval = 55 ms, AH interval = 90 ms and HV interval = -14 ms. The earliest anterograde ventricular and retrograde atrial electrograms were recorded at CS 3, 4 (Fig. 2A and 2B). The effective refractory period (ERP) and block cycle length (BCL) of the AP were as follows: anterograde ERP of the AP = 390 ms at a basic pacing CL (S1S1) of 600 ms, anterograde AP BCL = 450 ms, retrograde ERP of the AP = 280 ms at a basic pacing CL of 600 ms and retrograde AP BCL < 320 ms. Maximal preexcitation on 12-lead ECG was noted during rapid atrial pacing (Fig. 1B). A regular, narrow QRS tachycardia with a cycle length of 330 ms could be induced by extrastimulus pacing or burst pacing from the HRA or RV during the baseline state (Fig. 1C). The earliest activation site during the tachycardia was recorded in the distal CS (Fig. 2C). Prolongation of the tachycardia cycle length upon development of a left bundle branch block without any change in the atrial activation sequence supported the participation of the bypass tract in the reentrant circuit of the tachycardia. The patient consented to the use of the radial artery as the access site after a discussion with the operator regarding the potential benefits and risks of its use. After confirmation of a normal result of the Allen's test, cannulation of the left radial artery was performed and a 7-French ablation catheter with a 4 mm distal tip was advanced through the artery to map the mitral annulus. The earliest ventricular activation preceding the onset of the local V by 14 ms was recorded during the sinus rhythm, with the catheter positioned in the 2 o'clock region of the mitral annulus. The delta wave disappeared 3 s after the application of the RF energy to that site with a temperature of 55℃. The RF energy was delivered for 60 s. There was neither anterograde nor retrograde conduction through the AP thereafter. The patient remained free from any palpitations or pre-excitation on the 12-lead ECG during a 10-month post-ablation follow-up (Fig. 3).
DISCUSSION
This is the first report on the use of the radial artery as an access site for catheter ablation. Some electrophysiologists prefer transseptal catheterization in patients with left AV bypass tracts on the premise it avoids potential injuries to the aorta and aortic valve and makes mapping of the mitral annulus easier than the retrograde transaortic approach.7,8 However, it can cause potentially dangerous complications such as an aortic puncture or cardiac tamponade and requires special training.9 To avoid the use of additional instruments and potentially catastrophic side-effects, other electrophysiologists use the retrograde transaortic approach as an initial approach.10,11 However, access site-related complications such as hematomas, pseudoaneurysms, or arteriovenous fistulas, can occur with the retrograde transaortic approach.12,13 Although there are several types of percutaneous vascular closure devices to overcome these complications, a meta-analysis demonstrated the ineffectiveness of these devices in preventing vascular complications.14 To avoid complications related to the femoral artery access and to promote early ambulation, the radial artery is widely used in percutaneous coronary angiography and intervention.2-5
Because of the relatively small size of the radial artery and its predisposition to spasms, it has not been considered an adequate access site for interventionalelectrophysiology. With the increase in geriatric population with tachyarrhythmias, the number of patients with co-morbid conditions such as severe peripheral artery disease, aortic atherosclerosis, or tortuous aortas, will also increase, and the chance of encountering non-accessible femoral arteries will grow. In those situations, if electrophysiologists are not confident with a transseptal catheterization, the radial artery might be one of the last options to map the mitral annulus or left ventricle.
There are several methods to decrease the risks of radial arterial spasms. Generous use of antispastic agents, including the calcium-channel blocker, nicorandil, nitrate and the use of a small diameter mapping catheter, will lessen the development of spasms.15,16 As clockwise or counterclockwise rotation of the catheter will promote radial artery spasms, rapid mapping of the optimal site with minimal rotation of the catheter will reduce the risk of spasms.
In summary, we report a case of a manifest left free-wall AP, which was successfully ablated by a retrograde transaortic approach via a radial artery. The use of the radial artery in the catheter ablation should be considered when patients have severe peripheral artery disease or an aortic pathology precluding a femoral arterial access.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download