Abstract
Patients with neurofibromatosis-1 (NF-1) have increased susceptibility to a variety of malignancies. Here, we document a rare case of two separated ileal adenocarcinomas in NF-1. The adenocarcinomas were surrounded by a diffuse tubular adenomatous lesion of the mucosa, and ganglion cells were scattered in the NF background. We found this case meaningful for several reasons: two separated adenocarcinomas arising in an unusual ileal segment, the association with precancerous tubular adenoma, and the presence of ganglion cells, which suggests ganglioneuromatosis in NF-1.
There is an increased risk of malignant tumors in patients with neurofibromatosis 1 (NF-1) as compared to the normal population.1-3 Gastrointestinal epithelial tumors are rarely described with NF-1, and there have been no reports of two separated ileal adenocarcinomas until now.4,5 We report a patient with neurofibromatosis whose course was complicated by two separated ileal adenocarcinomas.
A 40-year-old male patient was hospitalized for intermittent abdominal pain spanning several months. He was diagnosed with von Recklinghausen's disease (VRN) at age 10, with neurofibromas involving the skin over the abdomen. His aunt had the same multiple, pigmented skin lesions, but without abdominal symptoms (no biopsy available). On physical examination, multiple cafe-au-lait spots, and numerous subcutaneous nodules were visible. Blood pressure was 140/100 mmHg. Laboratory findings were within normal limits. A barium enema showed a long segmental small bowel dilatation and multiple smooth nodular filling defects in the distal ileum (Fig. 1). A computed tomography (CT) of the abdomen revealed a long segmental dilatation of the distal ileal loop. The dilated small bowel contained a thickened wall representing smoothly thickened mucosa, markedly thickened submucosa, muscle layers, and serosa. Two large polypoid masses were noted in the distal ileum. These masses were mainly composed of thickened mucosa with lobular contour, which were markedly enhanced (Fig. 2). Innumerable small nodular lesions were scattered along the serosal surface of the small intestine and in the mesentery. The appendix was also diffusely thickened, especially in the submucosa, or muscle layer, with low attenuation. Because ileal malignancy was suspected, a segmental resection of the small intestine and colon was performed.
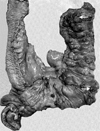
The specimens consisted of a small intestinal segment measuring 58 cm, a continuous colonic segment measuring 36 cm in length, and an appendix measuring 9 × 9 × 6 cm in size. Over 100 small conglomerated nodules on the serosal surface were situated along the mesenteric border of the ileum and colon. The opened specimen revealed two polypoid masses in the ileal segment (Fig. 3). The larger mass measured 23 cm distant from the ileocecal valve and 10 × 9 × 6 cm in size, and the smaller mass was 46 cm distant from the ileocecal valve and 8 × 7 × 5 cm in size. The surrounding small intestinal mucosa showed diffuse, irregularly elevated edematous lesions with pin point ulcerations. A cut section of bowel wall showed edematous submucosa and a thick fibrotic muscle layer (Fig. 4). The appendix showed flat mucosa with diffuse thickening of the muscle layer. The colonic wall did not show any polypoid mass lesions or transmural thickening.
Histologic evaluation of the two polypoid masses revealed well-to-moderately differentiated adenocarcinoma and surrounding tubulo-villous adenoma with neurofibromatosis background (Fig. 5A). In the larger mass, the carcinoma extended into the serosa and regional lymph nodes. In the smaller mass, the well differentiated adenocarcinoma was confined to the mucosa (Fig. 5B). The intervening edematous elevated lesion between the two polypoid masses showed a multifocal tubular adenoma with transmural neurofibromatous stroma. The mucosal tubular adenomatous glands extended into the submucosa without cytologic and structural atypism, resembling colitis cystica profunda (Fig. 6A). Each area of the involved ileal segment showed variable histological severity of neurofibromatosis. In the fully developed area, the mucosa showed individual and grouped ganglion cells with the background of neurofibromatosis (Fig. 6B). The muscularis mucosa was indistinguishable due to the proliferating neurofibromatous stroma. The ganglion cells were made more prominent in the immunohistochemical study by utilizing the S-100 protein, synaptophysin, and neuron specific enolase antibodies. The submucosa showed wavy spindle cell proliferation, with scattered mast cells, eosinophils, and some giant cells. This is similar to the findings in an inflammatory fibroid polyp. The muscle layers were split by intervening wavy spindle cells, which were accentuated with masson-trichrome stain. These cells were positive for S-100 protein and neurofilament antibodies, but negative for c-kit antibody. The intermuscular Auerbach plexus was hyperplastic, with a band-like structure, and the serosa showed many conglomerated nodules composed of wavy spindle cells. In the less developed area, we observed more nodular lesions rather than diffuse neurofibromatous proliferation. The appendiceal mucosa was atrophied, with diffuse neurofibromatous stroma in the submucosa and muscle layers. The colon showed mildly hypertrophied nerve fibers without NF stroma. We performed a genetic study for the ras oncogene. However the exon-21 hot spot was not mutated in either the tubular adenoma or adenocarcinoma.
Neurofibromatosis (NF) is an autosomal dominant disorder affecting all three germinal layers. Therefore, it can involve any organ system. It is not a single entity, but rather a group of heterogeneous, multi-systemic neurocutaneous disorders involving both neuroectodermal and mesenchymal derivatives. NF1 affects the gastrointestinal tract in up to 25% of patients, and stromal tumors have been reported as the most common lesion in gastrointestinal neurofibromatosis.2,3 Epithelial tumors, including colonic, pancreatic, and small intestinal adenocarcinomas, have also been reported in NF.4,5 Generally, small bowel tumors represent only 1 - 5% of all gastrointestinal malignancies, which is strikingly considering that the small intestine accounts for 75% of the length and 90% of the mucosal surface area of the gastrointestinal tract. Small intestinal adenocarcinomas are extremely rare, and there is evidence that small bowel adenocarcinomas arise from pre-existing adenomas in the same manner that they do in the large bowel. Although there have been several reported cases of small bowel adenocarcinoma developing in NF,6,7 we could not find a case of two separated ileal adenocarcinomas which developed in the background of tubulovillous adenoma. This finding lends support to the adenoma-carcinoma theory for small bowel adenocarcinoma in NF. Knowing the relationship between ganglion cells and NF, the presence of ganglion cells is frequently described in previous cases of intestinal neurofibromatosis.8-11 However, Chambonniere M-L8 mentioned ganglioneuromatosis can be distinguished from neurofibromatosis described in VRN which is characterized by interfasciculating spindle-cell bundles without associated proliferation of ganglion cells. There are two types of GNM defined by their degree of extension: 1) the pure mucosal form defined as nerve hyperplasia predominating in the intestinal mucosa associated with concomitant hyperplasia of the submucosal Meissner plexus; 2) the transmural form defined as transmural nerve hyperplasia with predominant involvement of the myenteric Auerbach plexus. In our case, the ganglion cells were mainly noted in the mucosa and submucosa. However ganglia in the intermuscular Auerbach plexus were hyperplastic and prominent. Therefore, we could not determine whether this case was purely mucosal, or transmural under Chambonniere's definition. We assert that an intermediate type should be established that combines the two known forms. In summary, we report for the first time a rare case of two separated ileal adenocarcinomas associated with tubulovillous adenoma and ganglioneuromatosis in neurofibromatosis.
Figures and Tables
Fig. 1
Barium enema reveals a long segmental small bowel dilatation and multiple smooth nodular filling defects in the distal ileum.
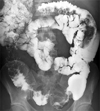
Fig. 2
Coronal reformatted CT image reveals thickened mucosa, submucosa and muscle layer and serosa layer in the distal ileum. Two polypoid masses are seen, representing well enhanced thickened mucosa. Innumerable small nodular lesions are scattered in mesentery.
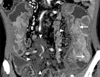
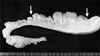
Fig. 4
Cross section of the intestinal wall revealed edematous submucosa and thick fibrotic muscle layer in the involved segment, which is contrasted with a continuous thin normal segment.
References
1. Korf BR. Malignancy in neurofibromatosis type 1. Oncologist. 2000. 5:477–485.
2. Joo M, Lee HK, Kim H, Kim MK, Chi JG. Multiple small intestinal stromal tumors associated with neurofibromatosis-1. Yonsei Med J. 2004. 45:564–567.
3. Hochberg FH, Dasilva AB, Galdabini J, Richardson EP Jr. Gastrointestinal involvement in von Recklinghausen's neurofibromatosis. Neurology. 1974. 24:1144–1151.
4. Jones TJ, Marshall TL. Neurofibromatosis and small bowel adenocarcinoma: an unrecognized association. Gut. 1987. 28:1173–1176.
5. Nelson AM. Small bowel adenocarcinoma associated with neurofibromatosis. Am J Gastroenterol. 1982. 77:149–151.
6. Perzin KH, Bridge MF. Adenomas of the small intestine: a clinicopathologic review of 51 cases and a study of their relationship to carcinoma. Cancer. 1981. 48:799–819.
7. Sellner F. Investigations on the significance of the adenoma-carcinoma sequence in the small bowel. Cancer. 1990. 66:702–715.
8. Chambonniere ML, Porcheron J, Scoazec JY, Audigier JC, Mosnier JF. Intestinal ganglioneuromatosis diagnosed in adult patients. Gastroenterol Clin Biol. 2003. 27:219–224.
9. Shekitka KM, Sobin LH. Ganglioneuromas of the gastrointestinal tract. Relation to Von Recklinghausen disease and other multiple tumor syndromes. Am J Surg Pathol. 1994. 18:250–257.
10. Snover DC, Weigent CE, Sumner HW. Diffuse mucosal ganglioneuromatosis of the colon associated with adenocarcinoma. Am J Clin Pathol. 1981. 75:225–229.
11. Shousha S, Smith PA. Colonic ganglioneuroma. Report of a case in a patient with neurofibromatosis, multiple colonic adenomas and adenocarcinoma. Virchows Arch A Pathol Anat Histol. 1981. 392:105–109.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download