Abstract
When conventional treatments of malignant pleural effusion, such as repeated thoracentesis, closed thoracotomy and pleurodesis by instilled sclerosing agents, are ineffective, there are few alternative therapies available. Our case involves a 47-year-old woman with uterine cervical carcinoma suffering from malignant pleural effusion. She presented with a chief complaint of severe dyspnea, and was classified as an Eastern Cooperative Oncology Group (ECOG) performance status of 4. Her underlying cervical carcinoma progressed despite various systemic chemotherapy regimens. In addition, pleural effusion persisted in spite of 4 weeks of drainage through the thoracotomy tube and talc pleurodesis. Under such circumstances, we attempted intrapleural chemotherapy with cisplatin plus cytarabine, which resulted in significant decrease of the pleural effusion. No serious systemic toxicities, including myelosuppression, were observed. As a result, the patient's dyspnea was relieved, and her ECOG performance status improved from 4 to 2. However, the thoracotomy tube was not removed due to subsequent iatrogenic pneumothorax. Pleural effusion did not recur for the 4 weeks leading up to her death.
Malignant pleural effusion (MPE) occurs frequently in patients with advanced malignancies, and is associated with a short life expectancy and may cause considerable morbidity.1 Most effusions do not respond to systemic chemotherapy, thus treatment is generally palliative. Conventional treatments of MPE include repeated thoracentesis, closed thoracotomy, pleurodesis (whose effect results from inflammation secondary to sclerosing agents), intrapleural chemotherapy with cytotoxic chemotherapeutic agents, and pleurectomy for palliation of symptoms or control of overall disease course.1 Of these treatments, talcation is now the treatment of choice for MPE, with all other therapies considered as alternatives.1 However, effective alternative treatment was not established for refractory MPE. Particularly, if the patient's general condition was poor, medical teams are apt to evaluate these patients without any viable treatment options to formulate a plan. We attempted to treat precisely this type of MPE patient with intrapleural cisplatin plus cytarabine. This treatment was selected based on known effectiveness and safety of intrapleural chemotherapy in patients with previously untreated MPE from various kinds of carcinomas.
In February of 2004, a 47-year-old woman with underlying uterine cervix carcinoma was admitted to our hospital with a 3 week history of severe dyspnea. She was diagnosed with uterine cervical adenocarcinoma (stage IIb) in March of 2000. The patient had undergone concurrent chemoradiation therapy with 5-FU/cisplatin and achieved complete remission. Multiple lung metastases occurred in November of 2001, and she received the 2nd line chemotherapy with paclitaxel/ifosfamide, the 3rd line chemotherapy with etoposide, and the 4th line chemotherapy with BVM (bleomycin, vincristine, mitomycin-C) regimens. However, the lung metastases progressed, and brain metastases developed newly after 2 cycles of BVM chemotherapy in January of 2003. No further systemic chemotherapy was performed.
The patient looked acutely ill on presentation, and was tachypneic (30/min). All other vital signs were within normal limits. Her Eastern Cooperative Oncology Group (ECOG) performance status was 4. No jugular venous distention was appreciated on physical exam. Breathing sounds decreased in the left upper lung field, with dullness on percussion. Chest CT scan (Fig. 1) and chest x-ray (Fig. 2) revealed a large amount of left pleural effusion and multiple lung metastases. Aside from a moderate hyponatremia (128 mEq/L), the complete blood cell count and serum chemistries were within normal limits. Liver and renal functions were normal. Cytology report of the pleural effusion revealed adenocarcinoma, suggesting a malignant pleural effusion secondary to uterine cervical cancer.
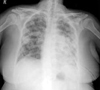
Closed thoracotomy was performed on the day after admission. 1,450 mL of pleural fluid was drained through the thoracotomy tube on day one. A daily median amount of 320 mL (range, 110 - 440) was drained for 25 days consecutively. Because at that time, the patient refused any further invasive treatments, delayed pleurodesis with 2.0 g of talc slurry was performed on day 26 of thoracotomy. However, pleural effusion did not decrease (Fig. 3).
After the patient gave written informed consent, she received intrapleural chemotherapy on day 32 of thoracotomy with cisplatin 100 mg/m2 and cytarabine 1,200 mg/m2 mixed into 250 mL normal saline. The patient was given pretreatment hydration with 2 L of normal saline and furosemide. She also received premedication 30 minutes prior to cisplatin chemotherapy, including IV infusion of granisetron (3 mg) and dexamethasone (10 mg). The mixed chemotherapeutic agents were infused through the thoracotomy tube, and a change in position was performed every 15 to 20 minutes. The instilled drugs were drained 4 hours later.
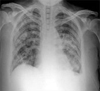
Since day 2 of intrapleural chemotherapy, daily drainage of pleural fluid decreased dramatically to a median amount of 110 mL (range, 20 - 240) until day 18 of intrapleural chemotherapy. Its daily drainage further decreased to less than 100 mL over the last 6 days (Fig. 4). This minimal pleural fluid reaccumulation was equivalent to partial response, defined as 75% or greater reduction of pleural effusion compared to baseline pretreatment chest radiograph, as noted in a previous, intrapleural chemotherapy trial.2 Toxicity consisted of grade 1 nausea and grade 2 pleuritic chest pain according to the National Cancer Institute's Common Toxicity Criteria version 3.0.3 No myelosuppression was observed.
We were unable to remove thoracotomy tube due to iatrogenic pneumothorax, which developed during exchange of the subclavian catheter on day 9 of intrapleural chemotherapy. However, her dyspnea due to malignant pleural effusion was relieved significantly due to intrapleural chemotherapy. ECOG performance status was improved from 4 to 2. Pleural effusion did not recur for the 4 weeks leading up to her death.
Systemic chemotherapy is generally disappointing for the control of malignant pleural effusions. When the underlying malignancy is chemo-sensitive, systemic chemotherapy might be the treatment of choice for malignant pleural effusion. Otherwise, tube thoracotomy with subsequent pleurodesis has traditionally been recommended.1 A number of sclerosing agents, such as tetracycline, bleomycin, and talc, were used for MPE.1 Talc is now the standard agent for sclerosing the pleural space in patients with malignant pleural effusions although fever is a relatively common adverse effect.1
In contrast to traditional sclerosing agents, intrapleural chemotherapy has the potential advantage of treating the underlying malignancy in addition to providing local control of MPE.2 Several chemotherapeutic agents have been tested, including doxorubicin,4 mitoxantrone,5 paclitaxel,6 and cisplatin plus cytarabine.2,7,8 Cisplatin has been widely used as an agent for intracavitary chemotherapy and has been shown to be safe and effective.9
On the basis of in vitro results demonstrating cisplatin and cytarabine were synergistic,10 Markman et al. conducted phase I and II studies of intrapleural chemotherapy with cisplatin plus cytarabine.7 Subsequently, in 1994 the Lung Cancer Study Group evaluated the effectiveness of intrapleural cisplatin plus cytarabine in patients with previously untreated MPE from various malignancies, such as lung, breast, ovarian, pleural, esophageal, pancreas, and stomach cancers, resulting in good response.2 Guided by these studies, we administered intrapleural chemotherapy in a uterine cervical cancer patient with refractory MPE. Intrapleural chemotherapy with palliative aim had not been previously tested in this type of patient. We observed this regimen, in a heavily-treated and exhausted patient, which resulted in a dramatic decrease of pleural effusion and subsequently in relief of patient symptoms.
Although we did not evaluate pharmacokinetics in this case, previous studies suggest intracavitary chemotherapy can lead to higher intracavitary concentration of drugs than systemic chemotherapy,11,12 which might induce good response. Cisplatin had been effective in this patient when she was first diagnosed with uterine cervical cancer, and readministration of this active agent might contribute to a good response. Otherwise, successful response in this case might be due to synergistic, cytotoxic effect of the two drugs.10
In conclusion, intrapleural chemotherapy with cisplatin plus cytarabine can be considered as palliative treatment for intractable malignant pleural effusion, particularly if the patient has a poor performance status and has no other available options.
Figures and Tables
Fig. 1
Chest CT scan performed on admission. Large amount of pleural effusion in the left lung and multiple lung metastases were observed.
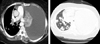
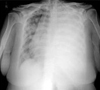
Fig. 2
At the time of admission. Huge amount of pleural effusion in the left lung and the deviation of the heart and the trachea were noted.
References
1. American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000. 162:1987–2001.
2. Rusch VW, Figlin R, Godwin D, Piantadosi S. Intrapleural cisplatin and cytarabine in the management of malignant pleural effusions: a Lung Cancer Study Group trial. J Clin Oncol. 1991. 9:313–319.
3. Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003. 13:176–181.
4. Masuno T, Kishimoto S, Ogura T, Honma T, Niitani H, Fukuoka M, et al. A comparative trial of LC9018 plus doxorubicin and doxorubicin alone for the treatment of malignant pleural effusion secondary to lung cancer. Cancer. 1991. 68:1495–1500.
5. Aasebø U, Norum J, Sager G, Slørdal L. Intrapleurally instilled mitoxantrone in metastatic pleural effusions: a phase II study. J Chemother. 1997. 9:106–111.
6. Perng RP, Chen YM, Wu MF, Chou KC, Lin WC, Liu JM, et al. Phase II trial of intrapleural paclitaxel injection for non-small-cell lung cancer patients with malignant pleural effusions. Respir Med. 1998. 92:473–479.
7. Markman M, Cleary S, King ME, Howell SB. Cisplatin and cytarabine administered intrapleurally as treatment of malignant pleural effusions. Med Pediatr Oncol. 1985. 13:191–193.
8. Aitini E, Cavazzini G, Pasquini E, Rabbi C, Colombo F, Cantore M, et al. Treatment of primary or metastatic pleural effusion with intracavitary cytosine arabinoside and cisplatin. A phase II study. Acta Oncol. 1994. 33:191–194.
9. Markman M, Cleary S, Pfeifle C, Howell SB. Cisplatin administered by the intracavitary route as treatment for malignant mesothelioma. Cancer. 1986. 58:18–21.
10. Vadi H, Drewinko B. Kinetics and mechanism of the 1-beta-D-arabinofuranosylcytosine-induced potentiation of cis-diamminedichloroplatinum(II) cytotoxicity. Cancer Res. 1986. 46:1105–1109.
11. Markman M. Intracavitary chemotherapy. Curr Probl Cancer. 1986. 10:401–437.
12. Howell SB, Pfeifle CE, Wung WE, Olshen RA. Intraperitoneal cis-diamminedichloroplatinum with systemic thiosulfate protection. Cancer Res. 1983. 43:1426–1431.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download