Abstract
Replication of the hepatitis B virus is suppressed by deficiency of the X protein. Although several molecules that block cellular targets of X protein reduce the production of hepatitis B virus progeny, the effect of a specific inhibitor of X protein on viral replication has not been investigated. To block X protein specifically, we adopted an intracellular expression approach using H7 single chain variable fragment (H7scFv), an antibody fragment against X protein. We previously demonstrated that cytoplasmic expression of H7scFv inhibits X protein-induced tumorigenicity and transactivation. In this study, intracellular H7scFv expression inhibits reporter gene transactivation but not viral replication determined by endogenous hepatitis B virus polymerase activity assay and real-time PCR. Our findings imply that intracellular expression of antibody fragment against X protein may not be an alternative therapeutic modality for inhibition of hepatitis B virus replication.
The control of replication of the hepatitis B virus (HBV) is important because HBV persistently infects more than 300 million people and is a principal cause of liver cancer.1 HBV has relaxed circular DNA containing four open reading frames encoding three structural proteins (the polymerase, core, and surface proteins) and one non-structural protein, X protein (HBx). Upon infection of hepatocytes, viral DNA is transported to the nucleus where it is converted to a covalently closed, circular, double-stranded DNA (cccDNA). Genomic and subgenomic viral transcripts, all of which utilize the same polyadenylation signal and contain the HBx gene sequence, are transcribed from cccDNA. The largest HBV transcript is encapsidated in cytoplasmic core particles, within which reverse transcription and DNA replication occur. Mature enveloped viral particles are eventually secreted from the infected cells.2
The importance of HBx in HBV replication has previously been reported. In HepG2 cells, the level of core particles of wild type virus was higher than that of a HBx deletional mutant.3,4 HBx can enhance viral replication in HBV transgenic mice, apparently through activation of viral gene expression.5 HBx has been shown to affect HBV replication through a proteasome-dependent pathway.6 Additionally, HBx acts on stored cytosolic calcium and activates Pyk2, which is critical for stimulation of HBV DNA replication in tissue culture.4 Similar to HBV, woodchuck hepatitis virus X protein is also required for viral infection in vivo.7
In addition to its role in viral replication, an oncogenic function of HBx has been demonstrated. HBx transactivates viral and host genes and stimulates the Ras-Raf-MEK-ERK signal transduction pathway.8,9 HBx expression in NIH-3T3 cells leads these cells to develop tumors in nude mice, and hepatocellular carcinomas develop in HBx transgenic mice.10,11 Previously, we showed that HBx-mediated tumorigenicity is suppressed by interfering with HBx function through intracellular expression of the H7 antibody single chain variable fragment (scFv) against HBx.12
Although several agents that block cellular targets of HBx reduce HBV replication, it remains an open question whether specific inhibitors of HBx may affect viral replication.4 In the present study, we investigated the effect of intracellular expression of H7scFv on HBV replication.
The eukaryotic expression vectors for H7scFv, with specificity to HBx, and for S2E1scFv, with specificity to surface protein of HBV, were described previously.12 Briefly, variable gene segments of the immunoglobulin heavy and light chain of H7 and S2E1 respectively were linked by a (GGGGS)3 flexible peptide linker. These constructs contain a Myc epitope adjacent to the N-terminus of each scFv that facilitates the detection of expressed proteins. Using NheI and XhoI sites, each myc tagged scFv gene was subcloned into pIRES-EGFP (Clonetech, Mountain View, CA, U.S.A.).
The HepG2-WT10 cell line that stably produces HBV (kindly provided by Dr. Cheng, Yale University School of Medicine, New Haven, Connecticut, U.S.A.) was cultured in DMEM (GIBCO BRL, MD) supplemented with 10% fetal bovine serum (GIBCO BRL, Carlsbad, California, U.S.A.).13 Cells (3 × 106) were seeded into a 10-cm-diameter culture dish and then transfected with 8 µg of empty vector (pCMV-Myc or pIRES-EGFP), H7scFv-expressing vector (pCMV-Myc-H7scFv or pIRES-H7scFv), or S2E1-expressing vector (pCMV-Myc-S2E1scFv or pIRES-S2E1) using Lipofectamine 2000 reagent (GIBCO BRL, Carlsbad, California, U.S.A.). All experiments were performed using both the pCMV vector series and pIRES vector series except the HBe Ag assay, which used the pIRES vector series.
HepG2-WT10 cells were transfected with H7sc Fv-expressing plasmid or control plasmid (pCMV-Myc-S2E1scFv or pCMV-Myc). After 48 hours, the cell lysates were separated on 12% SDS-PAGE, transferred to PVDF membranes and reacted with murine anti-Myc Ab for scFv or anti-tubulin Ab (Calbiochem, Darmstadt, Germany) as an internal control. Bound Ab was detected with horseradish peroxidase conjugated anti-mouse Ig antibody and an ECL detection system (Amersham, Piscataway, NJ, U.S.A.).
For the analysis of extracellular viral progeny DNA, HepG2-WT10 cells were transfected with pCMV-Myc, pCMV-Myc-H7scFv or pCMV-Myc-S2E1scFv and two days later the cell medium was harvested. The virus was precipitated by 10% polyethylene glycol 8000 in 0.5 M NaCl and 60 mM EDTA at 4℃ overnight. After centrifugation, the virus was resuspended in protein kinase buffer (PKB; 10 mM Tris-Cl (pH 7.8), 5 mM EDTA, 0.5% SDS) and digested with 100 µg/mL proteinase K (Sigma, St. Louis, MO, U.S.A.) for 1 hr at 37℃. DNA was purified by phenol/chloroform extraction and ethanol precipitation.
For the preparation of intracellular viral progeny DNA, HepG2-WT10 cells were lysed with 1 mL lysis buffer (10 mM Tris-Cl (pH 8), 1 mM EDTA, 1% NP-40, 50 mM NaCl) on ice for 10 min. The lysate was centrifuged for 2 min at 12,000g and the supernatant was treated with 10 U/mL DNase I (Takara, Kyoto, Japan) and 30 U/mL micrococcal nuclease (Calbiochem, San Diego, CA, U.S.A.), for 30 min at 37℃. A 4 concentration of PNE buffer (26% polyethylene glycol, 1.4 M NaCl, 40 mM EDTA) was added and the mixture was incubated for 1 hr on ice prior to centrifugation at 12,000g for 15 min. The DNA was isolated by digestion with 100 µg/mL proteinase K (Sigma, St. Louis, MO, U.S.A.) in PKB for 1 hr at 37℃, followed by a phenol/chloroform extraction and ethanol precipitation.
To analyze the effect of intracellular H7scFv on HBV replication, the amount of viral core DNA was measured using real-time PCR. Intracellular and extracellular core particles were obtained from HepG2-WT10 cells transiently expressing H7scFv or control S2E1. Cells were cultured for 48 hours prior to treatment with 100 µg/mL proteinase K (Sigma, St. Louis, MO, U.S.A.) for 1 hr at 37℃ and DNA extraction using phenol/chloroform and ethanol precipitation. Real-time PCR was performed on viral DNA as described previously.14 The amplification-detection was carried out in an ABI PRISM 7000 Sequence Detector (Applied Biosystem, Foster City, CA, U.S.A.).
Cytosolic core particles were isolated from equal numbers of HepG2-WT10 cells transfected with H7 scFv-expressing plasmid or control plasmid, and subjected to endogenous HBV polymerase activity assay (EPA) as previously described.15 Briefly, core particles were precipitated from cell lysates with 6.5% polyethylene glycol, incubated with EPA reaction buffer containing 10 µCi [α-32P]-dATP (3000 Ci/mmol) at 37℃ overnight, and resolved by gel electrophoresis and autoradiography.
HepG2-WT10 cells were transfected with H7sc Fv-expressing plasmid or control plasmid. After 48 hours in culture, supernatants were harvested and subjected to ELISA for HBeAg (DaiSorin S.P.A., Saluggia, Italy).
HepG2-WT10 cells grown on coverslips were fixed in 4% formaldehyde for 15 min and permeabilized in 0.2% Triton X-100. After 15 min the cells were incubated with murine monoclonal anti-Myc Ab (Clontech, Mountain View, CA, U.S.A.) overnight at 4℃, followed by incubation with FITC-conjugated anti-mouse IgG Ab (Jackson Immuno Research Laboratories Inc., West Grove, Pennsylvania, U.S.A.) at room temperature for 1 hr. Cells were examined with a fluorescence microscope (Olympus BX60, Tokyo, Japan).
HepG2-WT10 cells or HepG2 cells (2 × 105) were seeded into 24-well plates. After 16 hr, cells were co-transfected with pGL3-Control vector (Promega, Madison, WI, U.S.A.) containing the luciferase gene under the control of an SV40 enhancer/promoter and pCMV-Myc, pCMV-Myc-H7scFv or pCMV-Myc-S2E1scFv. After 24 hr or 48 hr, cells were harvested and lysed in 50 µL 1 × Passive Lysis Buffer (Promega, Madison, WI, U.S.A.). The cell lysate (5 µL) was mixed with 25 µL of Luciferase Assay Reagent II (Promega, Madison, WI, U.S.A.) and the light intensity was measured using a luminometer (Turner Designs, Sunnyvale, CA, U.S.A.).
HBx is expressed primarily in the cytoplasm of cells.16,17 Previously, we demonstrated that cytoplasmic expression of H7scFv, an antibody fragment specific for HBX, suppresses HBx-induced tumorigenicity and transactivation.12 Thus, to block HBx function in cells in which HBV replication occurs, H7scFv was expressed in the cytoplasm of HepG2-WT10 cells. As a control, S2E1scFv specific to the small surface glycoprotein (HBsAg) of HBV was used. S2E1scFv in the cytoplasm neither interacts with HBx nor interferes with HBx function in fibroblasts expressing HBx.12 It was reported by others that an antibody fragment specific to HBsAg interfered with HBsAg secretion when it was expressed in the ER, but not in the cytoplasm, of Huh-7 cells.18 We confirmed that intracytoplasmic expression of S2E1scFv has no effect on HBsAg production by HepG2-WT10 (data not shown). As shown in Fig. 1A, we observed an scFv band of approximately 30 kDa in each lysate from cells transfected with scFv-expressing plasmid. Cytoplasmic localization of H7scFv and S2E1scFv in transfected cells is shown in Fig. 1B.
Since the levels of HBeAg in patients' serum have shown correlation with viral replication, we next examined the effect of intracellular expression of H7scFv on the secretion of HBeAg by HepG2-WT10 cells.14 HBeAg production by Hep G2-WT10 cells transfected with plasmid expressing H7scFv was similar to that of controls transfected with empty vector or plasmid expressing S2E1scFv (Fig. 2). These results suggest that the suppression of HBx function has no effect on the production of HBeAg. According to Zhang's results using a HBx deletional mutant, HBeAg secretion by cultured cells was not affected by the expression of HBx, despite the reduced viral replication of the HBx deletional mutant.7
We analyzed the effect of intracellular H7scFv-expression on HBV replication using EPA. Cytosolic core particles were isolated from equal numbers of HepG2-WT10 cells transfected with H7scFv-expressing plasmid or control plasmid and subjected to EPA. The endogenous HBV polymerase activity obtained from cells expressing H7scFv was slightly decreased compared with cells containing empty vector, but similar to that of cells expressing S2E1scFv (Fig. 3). The relative level of endogenous HBV polymerase activity from cells expressing H7scFv to that from cells transfected with empty vector varied between experiments, with a maximum reduction of about 16% (data not shown). However, the endogenous HBV polymerase activity from cells expressing H7scFv was consistently similar to that from cells expressing S2E1scFv (data not shown). These results suggest that the expression of H7scFv inside cells to block HBx function may have no effect on the formation of cytoplasmic core particles.
To further analyze the effect of intracellular H7scFv-expression on HBV replication, the production of extracellular core particles and intracellular core particles was assessed by real-time PCR (Fig. 4). Serially diluted HBV DNA was amplified to give a standard curve, which was constructed by plotting the relative cycle threshold values against the known DNA copy number of each standard. The standard curve showed good regression (correlation coefficient r = 0.98, slope = -3.3). The copy number of each sample was calculated using the standard curve, and those of intracellular HBV core DNA were about 10 fold higher than that of extracellular HBV core DNA. As shown in Fig. 4, the copy numbers of both intracellular and extracellular HBV core DNA from cells expressing H7scFv were not reduced compared with those from cells containing control plasmids. These results indicated that intracellular H7scFv-expression has no effect on HBV replication.
To show the suppressive effect of intracellular H7scFv on HBx function in HepG2-WT10 cells, a luciferase reporter assay was performed. Luciferase activity increased with increased amount of transfected plasmid containing luciferase gene (Fig. 5A). Luciferase activity was higher in HepG2-WT10 cells than in HepG2 cells on both day 1 and day 2. A much higher luciferase activity was observed in HepG2-WT10 cells on day 2 than on day 1. These results suggest transactivation of the luciferase gene by viral proteins such as HBx. We next assessed luciferase activity in cells cotransfected with plasmid expressing scFv and plasmid containing the luciferase gene. As expected, luciferase activity was lower in HepG2-WT10 cells expressing H7scFv than in cells cotransfected with empty vector (Fig. 5). Unexpectedly, luciferase activity was also lower in HepG2-WT10 cells expressing S2E1scFv than in cells co-transfected with empty vector.
The present study was triggered by our previous work showing that intracellular H7scFv-expression suppresses HBx-mediated tumorigenicity.12 In this study, H7scFv expression inside HepG2-WT10 cells did not affect HBV replication even though it reduced gene transactivation in this cell line compared with cells transfected with control vector. Several intracellular Abs specific to viral proteins important in viral replication have been reported to affect viral lifecycles. Intracellular scFv against hepatitis B virus core protein suppresses viral replication in a HB611 cell line.19 With respect to human immunodeficiency virus, functional suppression of Vif protein, matrix protein p17, tat or gp120 by intracellular expression of a specific Ab fragment inhibits virus proliferation.20,21 However, in this study intracellular H7sc Fv targeting HBx did not affect viral replication.
HBx has been shown to enhance HBV replication in various experimental systems, but not in all systems. The viral progeny DNA level of a HBx deletional mutant was reduced to ~ 10% of that of wild type virus.4,7 Furthermore, HBx does not play a role in HBV replication in a Huh-7 cell-based system or a HBV transgenic mouse system.7,22,23 Thus, the significance of HBx in HBV replication is likely to depend on the experimental system, and it is possible that HBx is not critical in our experimental system.
We expressed H7scFv in the cytoplasm of Hep G2-WT10 cells. Considering that HBx is present in the nucleus at very low expression levels but becomes mostly cytoplasmic at increased expression levels,16 and that HBx likely interacts with or acts on the mitochondrial transition pore,24 the minor fraction of HBx in the nucleus or in mitochondria may not be affected by cytoplasmic H7scFv-expression. A recent report shows that HBx in association with DDB1 acts in the nucleus and stimulates HBV replication in HepG2 cells.25 Further study is needed to clarify whether intranuclear expression of H7scFv can inhibit HBV replication.
In this study we indirectly show the inhibitory effect of intracellular H7scFv expression on HBx function by the assay of reporter gene activity. The interaction of H7scFv with HBx inside cells and the inhibitory effect of H7scfv on HBx-induced gene activation was previously demonstrated.12 Interestingly, intracellular S2E1scFv expression also suppressed transactivation of the reporter gene in HBV-replicating HepG2-WT10 cells. A previous study reported that intracellular expression of S2E1scFv in NHBx1 cells that express HBx did not affect transactivation of a reporter gene.12 At present we cannot explain these findings, but we suggest one possibility. Since the target antigen of S2E1scFv is HBsAg, it is possible that S2E1scFv may recognize a polymerase and surface fusion protein, P-S fusion protein, a 43-kD protein containing epitopes of the N-terminus of polymerase and most parts of surface protein and which is encoded by a spliced RNA.26 Although transactivation of this P-S fusion protein has not yet been studied, P-S fusion protein cannot be ruled out based on the transcriptional activation of truncated middle surface (preS2/S) protein.27,28 In addition, at this point we cannot exclude the possibility that the expression of scFv itself affects transactivation of the reporter gene inside HepG2-WT10 cells.
In summary, we show that H7scFv-expression inside HBV-replicating cells inhibits reporter gene transactivation but not HBV replication. Our findings imply that the targeting of HBx by intracellular expression of an antibody fragment may not be effective for the control of HBV replication.
Figures and Tables
Fig. 1
Intracellular expression of H7scFv. (A) HepG2-WT10 cells were transiently transfected with H7scFv-expression plasmid or control plasmid (empty vector or S2E1scFv-expression plasmid). After 48 hours, the lysate of transfected cells was analyzed by Western blotting with anti-Myc-Ab (upper) and anti-tubulin Ab (lower). (B) HepG2-WT10 cells were transfected with H7scFv expression plasmid or control plasmid. One day after transfection, scFv protein in the cytoplasm was visualized by immunofluorescence microscopy using anti-Myc Ab and FITC-conjugated rabbit affinity purified Ab to mouse IgG.
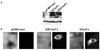
Fig. 2
HBeAg production was not affected by intracellular expression of H7scFv. Culture medium was harvested and analyzed by ELISA for HBeAg 48 hours after transfection of HepG2-WT10 cells with pIRES-H7scFv or control plasmid (pIRES-EGFP or pIRES-S2E1).

Fig. 3
Expression of intracellular H7scFv did not influence endogenous HBV polymerase activity. Cytoplasmic core particles were isolated from HepG2-WT10 cells transiently expressing H7scv or S2E1scFv, incubated with buffer containing [α-32P]dATP and visualized by gel electrophoresis and autoradiography. Relative band intensity was plotted.
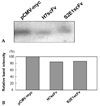
Fig. 4
Intracellular expression of H7scFv had no effect on HBV replication. HepG2-WT10 cells were transfected with pIRES-H7scFv or control plasmid. Forty-eight hours later, intracellular and extracellular core particles were isolated and viral DNA was amplified by real-time PCR. At the same time, serially diluted HBV plasmid samples were amplified for the construction of a standard curve (correlation coefficient r = 0.98, slope = -3.3). HBV DNA copy numbers were calculated using the standard curve and cycle threshold. The bar indicates the mean value of three different experiments. Similar results were obtained using the pCMV vector series.

Fig. 5
Intracellular H7scFv expression suppressed transactivation in HepG2-WT10 cells. (A) To demonstrate transactivation of a gene under the SV40 enhancer/promoter in HepG2-WT10 cells, luciferase activity was measured in cells transfected with the indicated amount of pGL3-Control vector. Increased luciferase activity was observed in HepG2-WT10 cells compared with HepG2 cells. The experiments were repeated three times and similar trends were observed in each case. (B) Luciferase activity was measured at the indicated time after co-transfection of HepG2 cells and HepG2-WT10 cells with pGL3-Control vector and H7scFv expression plasmid, S2E1scFv or empty vector. Relative luciferase activity shows the percentage of relative light units of the sample with respect to relative light units of WT10 cells transfected with empty vector. The mean percentage of triplicates with standard errors is shown. The experiments were repeated two times and similar trends were observed in each case.

References
1. Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988. 61:1942–1956.
2. Bouchard MJ, Schneider RJ. The enigmatic X gene of hepatitis B virus. J Virol. 2004. 78:12725–12734.
3. Melegari M, Scaglioni PP, Wands JR. Cloning and characterization of a novel hepatitis B virus x binding protein that inhibits viral replication. J Virol. 1998. 72:1737–1743.
4. Bouchard MJ, Wang LH, Schneider RJ. Calcium signaling by HBx protein in hepatitis B virus DNA replication. Science. 2001. 294:2376–2378.
5. Xu Z, Yen TS, Wu L, Madden CR, Tan W, Slagle BL, et al. Enhancement of hepatitis B virus replication by its X protein in transgenic mice. J Virol. 2002. 76:2579–2584.
6. Zhang Z, Protzer U, Hu Z, Jacob J, Liang TJ. Inhibition of cellular proteasome activities enhances hepadnavirus replication in an HBX-dependent manner. J Virol. 2004. 78:4566–4572.
7. Zoulim F, Saputelli J, Seeger C. Woodchuck hepatitis virus X protein is required for viral infection in vivo. J Virol. 1994. 68:2026–2030.
8. Doria M, Klein N, Lucito R, Schneider RJ. The hepatitis B virus HBx protein is a dual specificity cytoplasmic activator of Ras and nuclear activator of transcription factors. EMBO J. 1995. 14:4747–4757.
9. Benn J, Su F, Doria M, Schneider RJ. Hepatitis B virus HBx protein induces transcription factor AP-1 by activation of extracellular signal-regulated and c-Jun N-terminal mitogen-activated protein kinases. J Virol. 1996. 70:4978–4985.
10. Shirakata Y, Kawada M, Fujiki Y, Sano H, Oda M, Yaginuma K, et al. The X gene of hepatitis B virus induced growth stimulation and tumorigenic transformation of mouse NIH3T3 cells. Jpn J Cancer Res. 1989. 80:617–621.
11. Kim CM, Koike K, Saito I, Miyamura T, Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991. 351:317–320.
12. Jin YH, Kwon MH, Kim K, Shin HJ, Shin JS, Cho H, et al. An intracellular antibody can suppress tumorigenicity in Hepatitis B virus X-expressing cells. Cancer Immunol Immunother. 2006. 55:569–578.
13. Fu L, Cheng YC. Characterization of novel human hepatoma cell lines with stable hepatitis B virus secretion for evaluating new compounds against lamivudine- and penciclovir-resistant virus. Antimicrob Agents Chemother. 2000. 44:3402–3407.
14. Chen RW, Piiparinen H, Seppanen M, Koskela P, Sarna S, Lappalainen M. Real-time PCR for detection and quantitation of hepatitis B virus DNA. J Med Virol. 2001. 65:250–256.
15. Kim HY, Park GS, Kim EG, Kang SH, Shin HJ, Park S, et al. Oligomer synthesis by priming deficient polymerase in hepatitis B virus core particle. Virology. 2004. 322:22–30.
16. Henkler F, Hoare J, Waseem N, Goldin RD, McGarvey MJ, Koshy R, et al. Intracellular localization of the hepatitis B virus HBx protein. J Gen Virol. 2001. 82:871–882.
17. Sirma H, Weil R, Rosmorduc O, Urban S, Israel A, Kremsdorf D, et al. Cytosol is the prime compartment of hepatitis B virus X protein where it colocalizes with the proteasome. Oncogene. 1998. 16:2051–2063.
18. zu Putlitz J, Skerra A, Wands JR. Intracellular expression of a cloned antibody fragment interferes with hepatitis B virus surface antigen secretion. Biochem Biophys Res Commun. 1999. 255:785–791.
19. Yamamoto M, Hayashi N, Takehara T, Ueda K, Mita E, Tatsumi T, et al. Intracellular single-chain antibody against hepatitis B virus core protein inhibits the replication of hepatitis B virus in cultured cells. Hepatology. 1999. 30:300–307.
20. Chen SY, Bagley J, Marasco WA. Intracellular antibodies as a new class of therapeutic molecules for gene therapy. Hum Gene Ther. 1994. 5:595–601.
21. Goncalves J, Silva F, Freitas-Vieira A, Santa-Marta M, Malho R, Yang X, et al. Functional neutralization of HIV-1 Vif protein by intracellular immunization inhibits reverse transcription and viral replication. J Biol Chem. 2002. 277:32036–32045.
22. Blum HE, Zhang ZS, Galun E, von Weizsacker F, Garner B, Liang TJ, et al. Hepatitis B virus X protein is not central to the viral life cycle in vitro. J Virol. 1992. 66:1223–1227.
23. Reifenberg K, Nusser P, Lohler J, Spindler G, Kuhn C, von Weizsacker F, et al. Virus replication and virion export in X-deficient hepatitis B virus transgenic mice. J Gen Virol. 2002. 83(Pt 5):991–996.
24. Rahmani Z, Huh KW, Lasher R, Siddiqui A. Hepatitis B virus X protein colocalizes to mitochondria with a human voltage-dependent anion channel, HVDAC3, and alters its transmembrane potential. J Virol. 2000. 74:2840–2846.
25. Leupin O, Bontron S, Schaeffer C, Strubin M. Hepatitis B virus X protein stimulates viral genome replication via a DDB1-dependent pathway distinct from that leading to cell death. J Virol. 2005. 79:4238–4245.
26. Huang HL, Jeng KS, Hu CP, Tsai CH, Lo SJ, Chang C. Identification and characterization of a structural protein of hepatitis B virus: a polymerase and surface fusion protein encoded by a spliced RNA. Virology. 2000. 275:398–410.
27. Meyer M, Caselmann WH, Schluter V, Schreck R, Hofschneider PH, Baeuerle PA. Hepatitis B virus transactivator MHBst: activation of NF-kappa B, selective inhibition by antioxidants and integral membrane localization. EMBO J. 1992. 11:2991–3001.
28. Caselmann WH, Renner M, Schluter V, Hofschneider PH, Koshy R, Meyer M. The hepatitis B virus MHBst 167 protein is a pleiotropic transactivator mediating its effect via ubiquitous cellular transcription factors. J Gen Virol. 1997. 78(Pt 6):1487–1495.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download