Abstract
Autologous stem cell transplantation (ASCT) is commonly used in relapsed or refractory non-Hodgkin's lymphoma (NHL). Several trials report the role of ASCT for high risk patients. We evaluated the results and the prognostic factors influencing the therapeutic effects on the patients who were treated with high dose chemotherapy (HDC) and autologous peripheral stem cell transplantation. We analyzed the data of 40 cases with NHL who underwent ASCT after HDC. Twenty-four patients had high-risk disease, 12 cases sensitive relapse, and two cases resistant relapse or primary refractory each. The median age of patients was 34 years (range, 14-58 years). The median follow-up duration from transplantation was 16 months (range, 0.6-94 months). Estimated overall survival and progression-free survival at 5 years were 40% and 30%, respectively. Poor prognostic factors for survival included older age (≥ 45 years), poor performance status in all patient analysis, and a longer interval between first complete remission and transplantation in high risk patients. In high risk NHL patients, transplantation should be done early after first complete remission to overcome chemo-resistance.
The regimen of cyclophosphamide, doxorubicin, vincristine, and predisolone (CHOP) is the standard initial treatment for disseminated aggressive lymphoma in adults, and currently monoclonal antibody has become part of standard treatment in CD20+ non-Hodgkin's lymphoma (NHL).1,2 Conventional combination chemotherapy can induce a completion remission (CR) in about 60 percent of patients with disseminated intermediate-grade or high-grade NHL.3 However, sustained remission after the first line of therapy is only achieved in 40-50% of unselected patients.3 Other intensive third-generation regimens did not prove to be better than CHOP.4 Although a variety of salvage chemotherapy protocols have been developed, reported long-term survival rates range less than 10%.5 Since the 1980s, certain reports have indicated that high-dose chemotherapy (HDC) with autologous stem cell transplantation (ASCT) may be superior to conventional therapy in selected younger patients with aggressive lymphoma.6,7 At present, HDC with ASCT is the treatment of choice for patients with relapsed aggressive NHL.8 The role of HDC with ASCT as part of the initial therapy for high-risk patients is promising but not yet conclusively determined.9-15 Furthermore, recently reported and ongoing trials are studying the role of ASCT in patients with low-grade NHL.16,17
We report here a retrospective analysis of 40 patients who underwent autologous peripheral stem cell transplantation after HDC in Severance Hospital between 1996 and 2004.
A total of 40 patients diagnosed with NHL were included in this study. Criteria for ASCT were patients with any of the following conditions: those relapsed but chemo-sensitive, those who had not achieved CR but were chemo-sensitive, those who were relapsed and chemo-resistant; those who were primary refractory to salvage chemotherapy, or patients considered to have a high risk of relapse according to the International Prognostic Index (IPI). The high risk group had a lower chance of being cured with standard induction therapy and were defined as responding to first-line chemotherapy and by fulfilling at least one of the following criteria: stage III or IV,18 bulky disease or elevated lactate dehydrogenase (LDH).15 The high risk group included patients who achieved their first CR after induction chemotherapy and patients who failed to achieved CR after induction therapy but showed partial response (PR). Chemotherapy sensitivity was defined as a reduction in measurable disease with salvage chemotherapy that exceeded the PR criteria. Chemo-sensitive patients included sensitive-relapse patients who relapsed after induction therapy. Primary-refractory disease was defined as a stable or progressive disease documented at restaging immediately after the completion of induction therapy.19 Other inclusion criteria were as follows: diagnosis of NHL confirmed by histopathologist; age between 14 and 60 years; normal liver, kidney, heart, and pulmonary function on the basis of routine clinical and laboratory examinations; radionuclide ventriculography or echocardiography; lung-function tests; and an Eastern Cooperative Oncology Group performance status (ECOG) of 0 through 2. Informed consent was obtained from all patients.
As the first line treatment, 55% of the patients received CHOP, and response to first-line treatment was as follows: CR in 28 patients (70%), PR in 8 patients (20%) and no response in 4 patients (10%).
Autologous progenitor cells were mobilized with peripheral blood by using mobilization chemotherapy and granulocyte colony stimulating factor (G-CSF). Thirty-seven patients (92%) were conditioned with BEAM (carmustine, etoposide, cytarabine and melphalan) and 3 patients (8%) were conditioned with BEAC (carmustine, etoposide, cytarabine and cyclophosphamide). After conditioning regimen autologous stem cells were infused. Daily G-CSF 300 µg/m2/day subcutaneously starting d+5 and continuing until an absolute neutrophil count of > 1,000/µL for 3 consecutive days or > 10,000/µL. Patients received supportive care, including hydration, prophylactic oral antibacterial and antifungal treatment, and acyclovir was administered at 15 mg/kg/day from d-9 until neutrophil recovery. Intravenous globulin 500 mg/kg was administered at d-9, d-3, d+1 and the weekly until d+56 then biweekly until d+120. Patients were transfused with irradiated blood products to maintain a platelet count > 50,000/µL and hematocrit > 30%.
The clinical and radiological staging after HDC with ASCT was evaluated at 3 months. Complete remission was defined as complete tumor disappearance, and partial remission as the reduction of measurable disease by ≥ 50% of tumor area without the appearance of any new lesions. No response was defined as unmodified disease after the transplant or the appearance of new lesions.
After ASCT, engraftment was confirmed by an increase of WBC to more than 500/µL and an increase of platelet level to more than 50,000/µL.20 Toxicity was assessed using the World Health Organization toxicity criteria.
The survival curve was calculated according to the Kaplan-Meier method. Overall survival (OS) was calculated from the day of stem cells infusion to the date of death or last follow-up.21 Progression-free survival (PFS) was calculated from the day of stem cells infusion until the date of relapse or progression.21 In order to analyze which factors influence OS and PFS, clinical and laboratory factors at diagnosis and transplantation were included in the univariate analysis. Those factors with a statistically significant influence on OS or PFS determined by univariate analysis were included in a multivariate analysis. These variables are specified in Table 1 and 2. The log-rank test was used to estimate the differences in survival between groups according to the different covariates. All reported p values are two-sided and the value of p < 0.05 was considered significant. Factors that were predictive of OS and PFS in the univariate analysis were calculated using the Cox proportion hazards model for multivariate analysis. Statistical analyses were performed by SPSS version 12.0 (SPSS, Chicago, IL, USA).
Forty patients with NHL were analyzed. At the time of diagnosis, patients' ages ranged from 14-58 years, with a median age of 34 years. Of the pathologic diagnoses, diffuse large B cell were identified in 60% of patients, lymphoblastic B cell lymphoma in 17.5% and other diagnoses in 22.5% (Table 1). At the time of initial diagnosis, 98% had ECOG 0 or 1, 88% of patients had stage III or IV disease, and 21 (60%) of 35 assessable patients had an elevated LDH level. Ten (25%) patients had bulky disease at diagnosis (≥ 10 cm). Twenty-two patients (55%) received CHOP regimen for remission induction. Complete remission was achieved in 35 patients (87.5%), and of these, 14 patients (40%) relapsed before transplantation. Salvage chemotherapy included MiCMA (mitoxantrone, carboplatinum, methylpredisolone, cytarabine): 30%, ESHAP (etoposide, methylpredisolone, cytarabine, carboplantinum): 15%, HOAP-Bleo (adriamycin, vincristine, cytarabine, predisolone, bleomycin): 15%, IMVP-16 (ifosfamide, methotrexate, etoposide) and FND (fludarabine, mitoxantrone, dexamethasone).
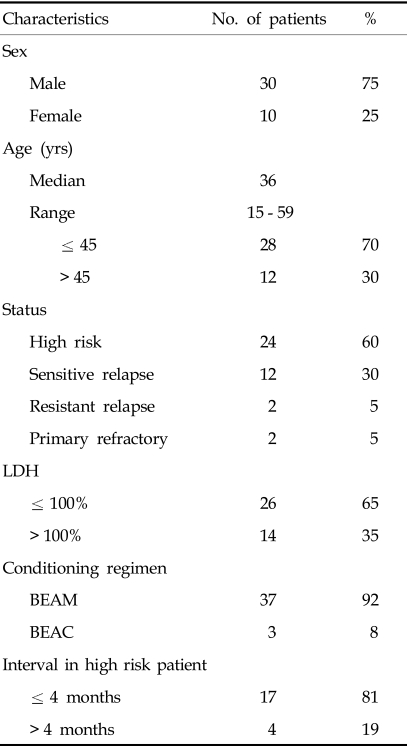
At the time of transplantation, patients' ages ranged from 15-59 years, with a median age of 36 years. We divided the 40 patients into 4 groups according to the disease status before transplantation; 24 patients with high risk disease, 12 with sensitive relapsed disease, 2 with resistant relapsed, and 2 in primary-refractory status. Nine (82%) of the sensitive relapsed patients and 2 (100%) of the resistant relapsed patients were in the high risk group at diagnosis (Table 2).
The median number of infused CD34+ cells was 12.5 × 106/kg (range, 4.0-543.0 × 106/kg). The median time to a WBC count > 500/µL was 9 days (range, 7-18 days) and to > 1000/µL, 10 days (range, 7-26 days). The median time to a platelet count of > 50,000/µL was 12 days (range, 0-27 days) and to 100,000/µL, 17 days (range, 8-186 days). Treatment-related death occurred in one patient (2.2%), as a result of veno-occlusive disease. Severe grade 3/4 mucositis and diarrhea were observed in 5% and 5% respectively, and grade 3/4 nausea, vomiting, liver toxicity in 2.5%, 2.5%, 2.5%, respectively.
The overall response rate to ASCT after 3 months was 70%. In high risk patients (21 patients in first CR after induction therapy, 3 patients in chemo-sensitive but not reach CR), 83.3% (20 cases) remained or achieved on CR, and 16.7% (four cases) had recurrence or not achieve CR. In patients transplanted at sensitive relapse (12 patients), 58.3% reached CR and of the patients with resistant relapse, 50% achieved CR. In the two patients that transplanted in refractory disease, one patient reached CR, and the other did not respond.
The median follow-up period from transplantation was 16 months (range 0.6-94 months). The median OS and PFS were 28 and 27 months, respectively. OS and PFS rate were 40.0% (95% CI: 30.0-50.0%), 30.0% (95% CI: 19.0-41.0%) at 5 years, and were 40.0% (95% CI: 30.0-50.0%) and 30.0% (95% CI: 19.0-41.0%) at 8 years, respectively (Fig. 1). The OS and PFS curves reached plateaus at 53 and 58 months after ASCT, respectively.
In high risk patients, the median PFS was 58.4 months (OS did not reach median) and, OS and PFS rates at 5 years were 52.8% (95% CI: 39.2-66.4%) and 37.8% (95% CI: 20.0-55.6%), respectively. In sensitive-relapse patients, median OS and PFS were 19.3 months and 4 months and, OS and PFS rates at 5 years were 24.3% (95% CI: 5.6-43.0%) and 25.0% (95% CI: 12.5-37.5%), respectively. In resistant-relapsed patients, the median OS and PFS were both 3.4 months. In primary refractory patients, median OS and PFS were both 2.7 months. The patients who were refractory had shorter OS and PFS than high risk patients and relapsed patients (OS: p = 0.03, PFS: p = 0.11). High risk patients and sensitive-relapse patients did not show significant difference in OS and PFS rates (OS: p = 0.28, PFS: p = 0.09) (Fig. 2. A and B). In sensitive-relapse patients, those who remained in CR 3 months after ASCT had better OS and PFS than who were recurred (OS: p = 0.03, PFS: p = 0.001).
The following characteristics were included in the analysis of prognostic factors: age, histology, Ann Arbor stage, bone marrow involvement, number of extra nodal sites, and performance status (ECOG) at diagnosis. Also included were serum concentration of LDH, age-adjusted-IPI, serum concentration of beta-2 microglobulin, serum concentration of albumin, the presence of B-symptom, and the presence of bulky mass (> 10 cm) at diagnosis and transplantation. Response to initial chemotherapy, number of prior chemotherapy regimens, chemotherapy sensitivity, disease status at transplantation and interval from diagnosis to transplantation were other prognostic factors considered.22 In the univariate analysis, 2 factors [Age at the diagnosis (≤ 45 years vs. > 45 years), ECOG at diagnosis (0 or 1 vs. 2)] for all patients were significant (Age at the diagnosis, OS: p = 0.01 and PFS: p = 0.01, ECOG, OS: p = 0.007 and PFS: p = 0.01). In multivariate analysis, age at diagnosis was significant value for OS (p = 0.042) and, ECOG at diagnosis was significant for PFS (p = 0.046). In high risk patients who achieved CR after induction therapy, the interval from first CR to transplantation was prognostic factor for OS (p = 0.048) (Fig. 3). The other factors were not significant for OS and PFS in survival analysis.
NHL, especially diffuse large cell lymphoma is highly chemo-sensitive malignancy. Treatment with conventional chemotherapy yields CR rates of 50-70% and disease-free survival (DFS) rates of about 50%. However, 50-60% of patients are refractory to initial therapy or relapse from CR.14 Under ordinary circumstances, less than 10% of patients with relapsed lymphoma can be cured with conventional salvage chemotherapy. HDC with ASCT have been reported to improve prognosis in this cohort of patients and widely used for patients with a sensitive relapse.23 For high risk patients who had achieved CR after four courses of induction treatment, the LNH87-2 study reported that HDC with ASCT benefits patients at higher risk according to the age- adjusted IPI who achieve CR after induction therapy.
In our study in Korea, the overall survival and progression free survival rates for the 40 patients of aggressive NHL were 53% and 52% at 2 years, respectively, and were 40% and 30% at 5 years and, 40% and 30% at 8 years, respectively. These are higher survival rates than those reported by Kim et al. for Koreans (OS and PFS at 2 years: 48% and 31%, respectively) and Shim et al. (OS and PFS at 2 years: 52% and 36%, respectively).24,25
In Western countries, the GEL-TAMO study showed the OS and DFS rates of 452 NHL patients treated with ASCT to be 53% and 43% at 5 years, and Dowling et al. reported a 5-year OS and PFS rates of 44% and 34% in 67 patients, respectively.26 The prognostic significance of a T-cell immunophenotype has been addressed and it is generally accepted that the T-cell immunophenotype confers an adverse prognosis, independent of IPI.27 T-cell NHL is rare in Europe and the United States, where it constitutes about 15-20% of aggressive lymphoma.28
In the Dowling et al. study, the proportion of the B-cell to T-cell subtype was 76%: 12%. On the other hand, T-cell NHL is more common in Taiwan (24.5%) and Japan (24.9%).28,29 In Korea the incidence of T cell lymphoma is assumed to be about 25-38% (in our study: 27.5%, in Hahn et al.'s study: 38%).30,31 We speculate that the lower survival rates of ASCT in our study were due to the higher frequency of the T cell subtype as compared to other studies in the Western countries. Rodriguez et al. suggested that the poor prognostic implication of the T-cell immunophenotype can be overcome with ASCT, and that future clinical trials are needed to determine whether ASCT either in the salvage setting or as frontline therapy can improve treatment outcome for T cell lymphoma.27
The PARMA study reported that the treatment of HDC with ASCT increases event-free and overall survival in patients with chemotherapy-sensitive NHL in relapse.8 We reported 5-year OS and PFS rates of 24.3% and 25.0% for sensitive relapse patients, respectively, and Mills et al. reported those with chemo-sensitive but relapsed disease as having an OS rate at 5 years of 32%.5 Even though high risk patients tended to show slightly longer survival time than the sensitive relapse patients, and much better survival than the resistant relapse patients, there was no statistically significant difference between the three groups. Further study will be needed with a larger number of patients.
In this study, the OS and PFS rates for high risk patients was 52.8% and 37.8% at 5 year, respectively. Santini et al. reported OS and PFS of 65% and 60% at 6 years for 63 patients who received VACOP-B (etoposide, doxorubicin, cyclophosphamide, vincristine, predisolone, bleomycin) for 12 weeks plus HDC with ASCT, independent of disease status at the end of VACOP-B.10 Twenty-one high risk patients in our study received ASCT as consolidation in the first CR after full-length induction treatment and showed 5-year OS and PFS rates of 46% and 25%, respectively. The LNH 87-2 study reported 8-year disease-free survival rates was 55% and 8-year OS rates was 64% in high risk NHL.9
In our study, the median interval between diagnosis and stem cell infusion in high risk patients was 28 weeks, whereas those of Hauioun et al. and Santini et al. were 19 and 18 weeks, respectively.9,10 Several reports suggest that ASCT is most effective when performed after maximal cytoreduction.32 Gisselbrecht et al. showed that the shortened standard induction therapy followed by HDC with ASCT has not shown to benefit event-free and overall survival rates, and Maurizio et al. showed that the abbreviated induction therapy and HCD with ASCT does not significantly improve survival in patients with high risk aggressive NHL.13,33 On the other hand, Milpied et al. reported that up-front intensive chemotherapy with ASCT is superior to CHOP for lymphoma patients under 60 years old who have high intermediate risk according to the age-adjusted-IPI. In the HOVON-40 trials, up-front high-dose, sequential chemotherapy with intensified CHOP and ASCT improved the duration of response and survival of poor risk aggressive NHL patients (OS at 4 years: 50%, disease-free survival at 4 years: 74%).34,35 Further clinical trial are needed to analyze the optimal intensive therapy regimes and the timing for transplantation to overcome chemotherapy resistance and acquire the adequate chemotherapy-based debulking.
There were three patients in our study who failed to achieve CR after induction therapy but were chemotherapy-sensitive. At a 5-year follow-up, two patients were alive without disease, but the other had died due to disease progression. In the study by Vose et al., patients who never achieved CR but who were still chemotherapy-sensitive had PFS and OS rates of 31% and 37% at 5 years, respectively.36 Rodriguez et al. reported OS rates at 5 years as 43% and disease free survival rates at 5 years as 63% for CR responders after ASCT in diffuse large B-cell NHL not achieving CR after induction therapy.37 These results pointed to the benefit of using HDC with ASCT in patients achieving PR after induction chemotherapy.36
For twenty-four DLBCL patients (60%) treated with ASCT, OS rates at 2 and 5 years was 43% and 28.7%, and PFS rates at 2 and 5 years was 47% and 31.3%, respectively. In our study, twelve DLBCL patients were transplanted as consolidation after CR, in which OS and PFS rates at 2 years were 68.7% and 78.5%. Mounier et al. reported OS and PFS in DLBCL patients treated by front-line ASCT after CR were 68% and 76% at 5 years respectively.38 Other 12 DLBCL relapsed/refractory patients showed OS and PFS were 20.8% and 16.6% at 2 years, respectively and same rate at 5 years in our study.
In the European CUP trial, HDC with ASCT was more effective than the standard treatment with regard to OS and PFS in relapsed patients with follicular lymphoma.16 Noel et al. reported that long-term and durable remission may be seen in selected patients with low-grade NHL, especially in those with a low IPI score and chemosensitive relapse.17 As of 30 July 2004, two of the three in our study were in sustained CR, whereas one patient had relapsed at 53 months after transplantation and died due to the disease progression. The data on myeloablative therapy followed by ASCT in follicular lymphoma are encouraging and further clinical trials for follicular lymphoma should reevaluate the role of ASCT.39
The outcome for patients with primary-refractory disease is very poor. Other studies' results were similar to ours, confirming that ASCT is not proper for these patients and that a new treatment strategy is needed for them. Such treatments may include standard or nonmyeloablative allogeneic stem cell transplantation even though the positive result in the T-NK cell lymphoma case had already been reported in our institution after the nonmyeloablative allogeneic transplantation.5,37,40
The finding that older age (> 45 years) and poor performance statuses are significant adverse prognostic factors in NHL is consistent with other studies.13,22,23 The interval between the first CR and transplantation also influences the OS in high risk patients who achieved CR after induction therapy. This suggests that high risk patients considered as candidates for HDT with ASCT should undergo transplantation early in the course of the disease after the first CR.23 The rationale for reducing the interval between CR and transplantation is to overcome chemotherapy resistance on a tight schedule, following one as shortly after the other as possible.35
In our study, two of the relapsed patients, all of the primary-refractory disease patients and three of the high risk patients received the involved field radiotherapy (IFR). Three of seven who received IFR remain alive in continuous CR at the last follow-up. Several recent studies have demonstrated the method of 'involved-field' radiation as a treatment modality in lymphoma to minimize disease bulk before transplants, to reduce relapse rates at sites of prior disease at the time of the transplant or after the transplant in either a CR state or persistent disease.41,42 Although few patients were irradiated in our study so as to allow a meaningful analysis, IFR should be considered in the design of future HDC with ASCT in aggressive NHL patients.
In conclusion, transplantation in high risk NHL patients who have achieved a prior response to complete standard induction treatment should be performed early to avoid chemo-resistance; and treatment with ASCT is not appropriate for primary-refractory patients, who should be treated by novel strategies.
References
1. Elias L, Portlock CS, Rosenberg SA. Combination chemotherapy of diffuse histiocytic lymphoma with cyclophosphamide, adriamycin, vincristine and prednisone (CHOP). Cancer. 1978; 42:1705–1710. PMID: 361209.

2. Coiffier B. State-of-the-art therapeutics: diffuse large B-cell lymphoma. J Clin Oncol. 2005; 23:6387–6393. PMID: 16155024.

3. Armitage JO. Treatment of non-Hodgkin's lymphoma. N Engl J Med. 1993; 328:1023–1030. PMID: 8450856.

4. Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, et al. Comparision of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993; 328:1002–1006. PMID: 7680764.
5. Mills W, Chopra R, McMillan A, Pearce R, Linch DC, Goldstone AH. BEAM chemotherapy and autologous bone marrow transplantation for patients with relapsed or refractory non-Hodgkin's lymphoma. J Clin Oncol. 1995; 13:588–595. PMID: 7884420.

6. Philip T, Armitage JO, Spitzer G, Chauvin F, Jagannath S, Cahn JY, et al. High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate-grade or high-grade non-Hodgkin's lymphoma. N Engl J Med. 1987; 316:1493–1498. PMID: 3295541.

7. Verdonck LF, Dekker AW, van Kempen ML, Punt K, van Unnik JA, van Peperzeel HA, et al. Intensive cytotoxic therapy followed by autologous bone marrow transplantation for non-Hodgkin's lymphoma of high-grade malignancy. Blood. 1985; 65:984–989. PMID: 3884064.

8. Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995; 333:1540–1545. PMID: 7477169.

9. Haioun C, Lepage E, Gisselbrecht C, Salles G, Coiffier B, Brice P, et al. Survival benefit of high-dose therapy in poor-risk aggressive non-Hodgkin's lymphoma: final analysis of the prospective LNH87-2 protocol-a groupe d'Etude des lymphomes de l'Adulte study. J Clin Oncol. 2000; 18:3025–3030. PMID: 10944137.

10. Santini G, Salvagno L, Leoni P, Chisesi T, De Souza C, Sertoli MR, et al. VACOP-B versus VACOP-B plus autologous bone marrow transplantation for advanced diffuse non-Hodgkin's lymphoma: results of a prospective randomized trial by the Non-Hodgkin's Lymphoma Cooperative Study Group. J Clin Oncol. 1998; 16:2796–2802. PMID: 9704732.

11. Kaiser U, Uebelacker I, Abel U, Birkmann J, Trumper L, Schmalenberg H, et al. Randomized study to evaluate the use of high-dose therapy as part of primary treatment for aggressive lymphoma. J Clin Oncol. 2002; 20:4413–4419. PMID: 12431962.

12. Krauter J, Gorlich K, Ottmann O, Lubbert M, Dohner H, Heit W, et al. Early autologous stem-cell transplantation versus conventional chemotherapy as front-line therapy in high-risk, aggressive non-Hodgkin's lymphoma: an Italian multicenter randomized trial. J Clin Oncol. 2003; 21:1255–1262. PMID: 12663712.
13. Gisselbrecht C, Lepage E, Molina T, Quesnel B, Fillet G, Lederlin P, et al. Shortened first-line high-dose chemotherapy for patients with poor-prognosis aggressive lymphoma. J Clin Oncol. 2002; 20:2472–2479. PMID: 12011124.

14. Hennessy BT, Hanrahan EO, Daly PA. Non-Hodgkin lymphoma: an update. Lancet Oncol. 2004; 5:341–353. PMID: 15172354.

15. Bertz H, Zeiser R, Lange W, Fetscher S, Waller CF, Finke J. Long-term follow-up after high-dose chemotherapy and autologous stem-cell transplantation for high-grade B-cell lymphoma suggests an improved outcome for high-risk patients with respect to the age-adjusted International Prognostic Index. Ann Oncol. 2004; 15:1419–1424. PMID: 15319249.
16. Harry C. High dose Therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin's lymphoma: results from the randomized European CUP trial. J Clin Oncol. 2003; 21:3918–3927. PMID: 14517188.
17. Laudi N, Arora M, Burns LJ, Miller JS, McGlave PB, Barker JN, et al. Long-term follow-up after autologous hematopoietic stem cell transplantation for low-grade non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2005; 11:129–135. PMID: 15682074.

18. National Cancer Institute sponsored study of classifications of non-Hodgkin's lymphomas: summary and description of a working formulation for clinical usage. The Non-Hodgkin's Lymphoma Pathologic Classification Project. Cancer. 1982; 49:2112–2135. PMID: 6896167.
19. Shipp MA, Abeloff MD, Antman KH, Carroll G, Hagenbeek A, Loeffler M, et al. International Consensus Conference on High-Dose Therapy with Hematopoietic Stem Cell Transplantation in Aggressive Non-Hodgkin's Lymphomas: report of the jury. J Clin Oncol. 1999; 17:423–429. PMID: 10458261.

20. Schmitz N, Linch DC, Dreger P, Goldstone AH, Boogaerts MA, Ferrant A, et al. Randomised trial of filgrastin-mobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet. 1996; 347:353–357. PMID: 8598700.
21. Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999; 17:1244–1253. PMID: 10561185.
22. A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993; 329:987–994. PMID: 8141877.
23. Caballero MD, Perez-Simon JA, Iriondo A, Lahuerta JJ, Sierra J, Marin J, et al. High-dose therapy in diffuse large cell lymphoma: results and prognostic factors in 452 patients from the GEL-TAMO Spanish Cooperative Group. Ann Oncol. 2003; 14:140–151. PMID: 12488306.

24. Kim YK, Lee JJ, Ahn JS, Yang DH, Byun JR, Cho SH, et al. High-dose therapy followed by autologous stem cell transplantation in non-Hodgkin's lymphoma; a single center experience. Korean J Hematol Stem Cell Trans. 2004; 9:12–18.
25. Shim BY, Lee MA, Byun JH, Roh SY, Song CW, Park JN, et al. High dose chemotherapy and autologous stem cell transplantation for poor risk and recurrent non-Hodgkin's lymphoma: a single-center experience of 50 patients. Korean J Intern Med. 2004; 19:114–120. PMID: 15366643.
26. Dowling AJ, Prince HM, Wirth A, Wolf M, Januszewicz EH, Juneja S, et al. High-dose therapy and autologous transplantation for lymphoma: The Peter MacCallum Cancer Institute experience. Intern Med J. 2001; 31:279–289. PMID: 11512599.

27. Rodriguez J, Caballero MD, Gutierrez A, Marin J, Lahuerta JJ, Sureda A, et al. High-dose chemotherapy and autologous stem cell transplantation in peripheral T-cell lymphoma: the GEL-TAMO experience. Ann Oncol. 2003; 14:1768–1775. PMID: 14630683.
28. Gisselbrecht C, Gaulard P, Lepage E, Coiffier B, Briere J, Haioun C, et al. Prognostic significance of T-cell phenotype in aggressive non-Hodgkin's lymphomas. Groupe d'Etudes des Lymphomes de l'Adulte (GELA). Blood. 1998; 92:76–82. PMID: 9639502.
29. The world health organization classification of malignant lymphomas in Japan: incidence of recently recognized entities. Lymphoma Study Group of Japanese Pathologists. Pathol Int. 2000; 50:696–702. PMID: 11012982.
30. Hahn JS, Lee S, Chong SY, Min YH, Ko YW. Eight-year experience of malignant lymphoma; survival and prognostic factors. Yonsei Med J. 1997; 38:270–284. PMID: 9409190.

31. Ko YH, Kim CW, Park CS, Jang HK, Lee SS, Kim SH, et al. REAL classification of malignant lymphomas in the Republic of Korea: incidence of recently recognized entities and changes in clinicopathologic features. Hematolymphoreticular Study Group of the Korean Society of Pathologists. Revised European-American lymphoma. Cancer. 1998; 83:806–812. PMID: 9708949.
32. Cuttica A, Zallio F, Ladetto M, Di Nicola M, Caracciolo D, Magni M, et al. Patients with high-risk aggressive lymphoma treated with frontline intensive chemotherapy and autografting: evidence of marked differences in outcome between patients with age-adjusted International Prognostic Index scores 2 and 3. Cancer. 2003; 98:983–992. PMID: 12942566.
33. Martelli M, Gherlinzoni F, De Renzo A, Zinzani PL, De Vivo A, Cantonetti M, et al. Early autologous stem-cell transplantation versus conventional chemotherapy as front-line therapy in high-risk, aggressive non-Hodgkin's lymphoma: an Italian multicenter randomized trial. J Clin Oncol. 2003; 21:1255–1262. PMID: 12663712.

34. Milpied N, Deconinck E, Gaillard F, Delwail V, Foussard C, Berthou C, et al. Initial treatment of aggressive lymphoma with high-dose chemotherapy and autologous stem-cell support. N Engl J Med. 2004; 350:1287–1295. PMID: 15044639.

35. van Imhoff GW, van der Holt B, Mackenzie MA, Van't Veer MB, Wijermans PW, Ossenkoppele GJ, et al. Impact of three courses of intensified CHOP prior to high-dose sequential therapy followed by autologous stem-cell transplantation as first-line treatment in poor-risk, aggressive non-Hodgkin's lymphoma: comparative analysis of Dutch-Belgian Hemato-Oncology Cooperative Group Studies 27 and 40. J Clin Oncol. 2005; 23:3793–3801. PMID: 15809447.

36. Vose JM, Zhang MJ, Rowlings PA, Lazarus HM, Bolwell BJ, Freytes CO, et al. Autologous transplantation for diffuse aggressive non-Hodgkin's lymphoma in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol. 2001; 19:406–413. PMID: 11208832.

37. Rodriguez J, Caballero MD, Gutierrez A, Solano C, Arranz R, Lahuerta JJ, et al. Autologous stem-cell transplantation in diffuse large B-cell non-Hodgkin's lymphoma not achieving complete response after induction chemotherapy: the GEL/TAMO experience. Ann Oncol. 2004; 15:1504–1509. PMID: 15367411.

38. Mounier N, Gisselbrecht C, Briere J, Haioun C, Feugier P, Offner F, et al. Prognostic factors in patients with aggressive non-Hodgkin's lymphoma treated by front-line autotransplantation after complete remission: a cohort study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2004; 15:2826–2834. PMID: 15254050.

39. Buske C, Dreyling M, Unterhalt M, Hiddemann W. Transplantation strategies for patients with follicular lymphoma. Curr Opin Hematol. 2005; 12:266–272. PMID: 15928482.

40. Cheong JW, Kim JS, Jang JH, Suh HC, Lee ST, Kim HO, et al. Nonmyeloablative stem cell transplantation in a patient with NK/T cell lymphoma. Korean J Hematol Stem Cell Trans. 2001; 6:229–236.
41. Wadhwa P, Shina DC, Schenkein D, Lazarus HM. Should involved-field radiation therapy be used as an adjunct to lymphoma autotransplantation? Bone Marrow Transplant. 2002; 29:183–189. PMID: 11859389.

42. Mundt AJ, Williams SF, Hallahan D. High dose chemotherapy and stem cell rescue for aggressive non-Hodgkin's lymphoma: pattern of failure and implications for involved-field radiotherapy. Int J Radiat Oncol Biol Phys. 1997; 39:617–625. PMID: 9336141.

Fig. 2
(A) Overall survival rates according to disease status at transplantation. High risk vs. Resistant relapse, p = 0.37; Sensitive relapse vs. Resistant relapse, p = 0.68. (B) Progression free survival rates according to disease status at transplantation. High risk vs. Resistant relapse, p = 0.59; Sensitive relapse vs. Resistant relapse, p = 0.85.
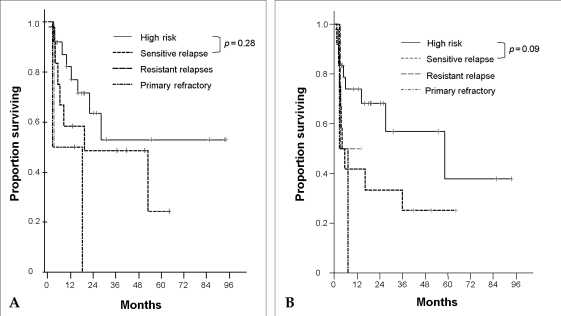
Fig. 3
Overall survival rates according to interval from CR to transplantation in high risk patients.
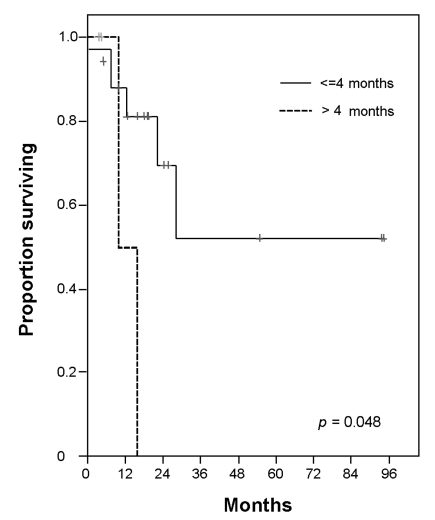
Table 1
Patient Characteristics at Diagnosis
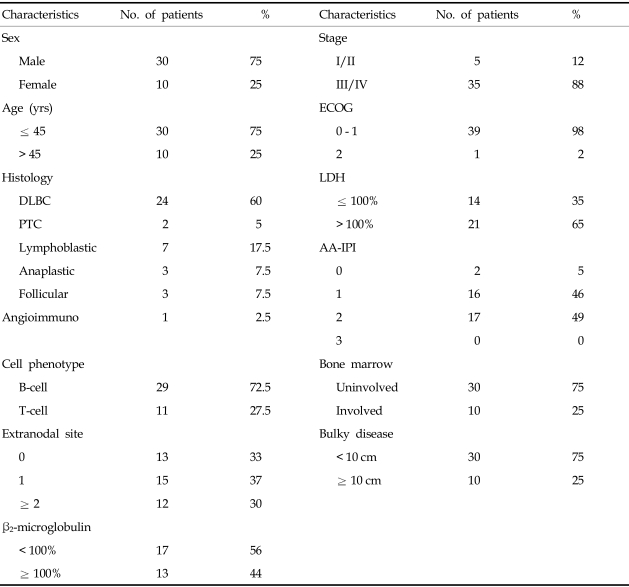
DLBC, diffuse large B cell lymphoma; PTC, peripheral T cell lymphoma; Lymphoblastic, lymphoblastic lymphoma; Anaplastic, anaplastic T cell lymphoma; Follicular, follicular lymphoma; Angioimmuno, angioimmunoblastic T-cell lymphoma; ECOG, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; AA-IPI, age-adjusted International Prognostic Index.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download