Abstract
The human telomerase reverse transcriptase (hTERT) promoter can be used for the tumor-specific expression of transgenes in order to induce selective cancer cell death. The hTERT core promoter is active in cancer cells but not in normal cells. To examine whether the combination of TNF-related apoptosis inducing ligand (TRAIL) treatment and cancer cell-selective expression of the TRAIL-death receptor could induce cell death in TRAIL-resistant cancer cells, we generated a death receptor-4 (DR4)-expressing adenovirus (Ad-hTERT-DR4), in which the expression of DR4 is driven by the hTERT promoter. Upon infection, DR4 expression was slightly increased in cancer cell lines, and cell death was observed in TRAIL-resistant cancer cell lines but not in normal human cells when DR4 infection was combined with TRAIL treatment. We also generated an adenovirus that expresses a secretable isoleucine zipper (ILZ)-fused, extracellular portion of TRAIL (Ad-ILZ-TRAIL). In cells infected with Ad-ILZ-TRAIL, TRAIL was expressed, secreted, oligomerized and biologically active in the induction of apoptosis in TRAIL-sensitive cancer cells. When Ad-hTERT-DR4 infected TRAIL-resistant HCE4 cells and Ad-ILZ-TRAIL infected TRAIL-resistant HCE7 cells were co-cultured, cell deaths were evident 24 h after co-culture. Taken together, our results reveal that the combination of TRAIL and cancer cell-specific expression of DR4 has the potential to overcome the resistance of cancer cells to TRAIL without inducing significant cell death in normal cells.
Human telomerase is a ribonucleoprotein enzyme that is important for the maintenance of telomeric structures at the ends of chromosomes.1-3 Telomerase activity is closely associated with the expression of human telomerase reverse transcriptase (hTERT). The expression of hTERT RNA is detected at high levels in tumor tissues and tumor-derived cell lines but not in normal adjacent tissues or primary cells.4-6 A 5' flanking region of 341 bp in the hTERT gene was identified as the minimal promoter region, containing elements responsible for its activity.7,8 The differential activity of this region in normal cells and cancer cells opens the possibility of utilizing the hTERT promoter for the selective expression of apoptosis-inducing genes only in cancer cells.
TNF-related apoptosis inducing ligand (TRAIL) is a promising agent for development as a cancer therapeutic9 because it appears to specifically kill transformed and cancer cells, whereas most normal cells appear to be resistant to TRAIL.10,11 TRAIL induces apoptotic cell death upon binding to either of two pro-apoptotic TRAIL receptors, TRAIL R1 (DR4)12 or TRAIL R2 (KILLER/DR5).13 Although TRAIL has the potential to specifically kill cancer cells, many cancer cells are TRAIL-resistant.14,15
Therefore, we performed this study to examine whether the combination of TRAIL treatment and cancer cell-selective expression of TRAIL death receptor DR4 could induce cell death in TRAIL-resistant cancer cells. We generated an adenovirus expressing DR4 under the control of the hTERT promoter to express DR4 selectively in cancer cells. Here, we show that the hTERT promoter is active in a variety of human cancer cell lines but not in normal primary human keratinocytes, and further that the tumor-specific expression of DR4 induces apoptosis in TRAIL-resistant cancer cells when combined with TRAIL.
Normal human primary cervical epithelial cells that were kindly provided by Dr. Young-Tae Kim (Department of Obstetrics and Gynecology, Yonsei University College of Medicine) were grown in Keratinocyte-SFM medium (Gibco BRL, Rockville, MD, USA) containing bovine pituitary extract (40 µg/mL) and epidermal growth factor (1 ng/mL). Cancer cell lines were grown as previously described.14,16
Cells were treated with 100 ng/mL final concentration of TRAIL17 for 2 h. The irreversible pan caspase inhibitor z-VAD-fmk (R&D Systems, Minneapolis, MN, USA) was used at a final concentration of 20 µM and was added 2 h prior to the addition of TRAIL.
The hTERT core promoter7 was amplified using genomic DNA as a template. For the amplification, the following primer pairs were used: Sense primer, 5'-GAAGATCT GGAGAGCTGCGCTGT-3' and anti-sense primer, 5'-GAAGATCTAAGCTT GCCAGGGCTT-3'. The amplified (301 bp) hTERT core promoter was cloned into the pGL3-basic (Promega, Madison, WI, USA) vector and was named pGL3-hTERT.
HCE4, CasKi, HCT116-neo, HCT116-E6, or normal human primary cervical epithelial cells were transiently transfected with pGL3-basic as a negative control, pGL3-control as a positive control, or pGL3-hTERT using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Briefly, 5 × 105 cells seeded in a 6-well plate were exposed to transfection mixtures containing 1.8 µg of the reporter plasmids and 0.2 µg of pCMV-β-galactosidase (BD Bioscience Clontech, Palo Alto, CA, USA) for 5 h at 37℃. Then, 3 mL of growth media were added to the cells, followed incubation for an additional 16 h. The cells were harvested 48 h after transfection. A luciferase ssay was performed according to the manufacturer's protocol (Promega). A β-galactosidase assay was performed to standardize transfection efficiency. In cells infected with adenovirus, infections were carried out 24 h after transfection.
To make an adenovirus expressing full length DR4 under the control of the hTERT core promoter, the pShuttle-CMV (Stratagene, La Jolla, CA, USA) vector was used. To obtain DR4 expression driven by the hTERT core promoter, the CMV promoter in pShuttle-CMV was replaced by a PCR amplified hTERT core promoter (pShuttle-hTERT) and then full length DR4 cDNA was inserted into the pShuttle-hTERT (pShuttle-hTERT-DR4) vector. Recombinant plasmid was generated in BJ5183 E. coli by co-transforming PmeI-digested pShuttle-hTERT-DR4 and pAdEASY.18 The recombinant plasmid was digested with PacI and was used for transfection into 293 cells using Lipofectamine 2000 (Invitrogen). Approximately 7 days after transfection, the cells were harvested, and the viruses were amplified and purified as previously described.19 A TRAIL-expressing adenovirus was made using the extracellular portion of TRAIL (Aa 95 to 281), which can be secreted and self-oligomerized. A DNA fragment that encodes the secretion signal from the V-J2-C region of the mouse Ig κ chain signal peptide, isoleucine zipper (ILZ) fragment that was made by annealing two synthetic oligonucleotides (5'-GGGGTACCATGAAGCAGATCGAGGACAAAATT GAGGAAATCCTGTCCAAGATTTACCACATCGAGAACGAGATCGCCCGGATTAAGAAACTCATTGGCGAGAAGCTTGGG-3' and 5'-CCCAAGCTTCTCGCCAATGAGTTTCTTAATCCGGGCGATCTCGTTCTCGATGTGGTAAATCTTGGACAGGATTTCCTCAATTTTGTCCTCGATCTGCTTCATGGTACCCC-3') for self-oligomerization20 and was sequentially inserted into the pAdTRACK/CMV vector. An adenovirus expressing the E6 ORF of human papillomavirus (HPV) type 16 was generated (Ad-E6). An adenovirus expressing green fluorescence protein (GFP) was also generated and used as a control virus (Ad-GFP). The infectivity of viruses was checked by the observation of green fluorescence within infected cells using fluorescence microscopy. To confirm the oligomerization of TRAIL, ethylene glycol bis-(succinic acid) (EGS, final 1 mM; Sigma, St. Louis, MO, U.S.A.) was used as a cross-linker to maintain oligomeric structure during denaturing SDS-PAGE analysis.
Western blot analysis was carried out as previously described.14 Blotted membranes were immunostained with anti-DR4 (1:500, Pharmingen, San Diego, CA, U.S.A.), anti-caspase-3 (E-8, 1:200; Santa Cruz, Santa Cruz, CA, U.S.A.), anti-PARP (1: 1,000; Cell Signaling Technology, Inc., Beverly, MA, U.S.A.), anti-TRAIL (1:500; PeproTech Inc., Rocky Hill, NJ, U.S.A.), or anti-α-tubulin (1:500; Oncogene Science, Cambridge, MA, U.S.A.).
Cells were harvested after the indicated treatments and time points, stained with propidium iodide, and analyzed by flow cytometry for sub-G1 content as previously described.21 Cell sorting was performed on a BD FACSCalibur (Becton Dickinson, Boston, MA, U.S.A.).
To examine the promoter activity of the hTERT gene in normal human cervical keratinocytes or in a TRAIL-resistant human esophageal cancer cell line, HCE4, a luciferase activity assay was performed. Transfection of pGL3-hTERT into HCE4 cells yielded a significant increase in promoter activity compared to the transfection of the pGL3 basic control vector (Fig. 1B). Transfection of the same construct into normal keratinocytes resulted only in background levels of luciferase activity (Fig. 1A). The E6 protein from HPV type 16 transcriptionally transactivates the hTERT promoter;1 expectedly, hTERT promoter activity was markedly increased in Ad-E6 infected cells (Fig. 1). These results suggest that the hTERT promoter is active only in cancer cells and that the presence f E6 protein could augment hTERT promoter activity.
We generated an adenovirus expressing the isoleucine zipper (ILZ)-fused extracellular portion of TRAIL (Ad-ILZ-TRAIL, Fig. 2A). To confirm the expression, secretion, and oligomerization of TRAIL, TRAIL-resistant HCE4 cells were infected with Ad-ILZ-TRAIL. Two days after infection, Western blot analysis was performed using cell extracts and the media. In the cell extracts, both un-processed and processed forms of TRAIL were present, but in the media only the processed form of the expressed TRAIL was present (Fig. 2B). The oligomerization of the secreted TRAIL was confirmed by cross-linking followed by Western blot analysis (Fig. 2C). The biological activity of TRAIL was examined using TRAIL-sensitive SW480 colon cancer cells. The cells infected with Ad-ILZ-TRAIL were morphologically apoptotic (Fig. 2D, TR). Procaspase-3 was activated and PARP was cleaved in the cells infected with Ad- ILZ-TRAIL but not in the presence of z-VAD-FMK (Fig. 2E. lane 2 vs. 4). Together, these data suggest that TRAIL expressed from the adenovirus is secreted, oligomerized, and biologically active.
An adenovirus that expresses DR4 under the control of the hTERT core promoter was generated (Fig. 3A). When infected into HCE4 or CasKi cells, the expression level of DR4 was slightly increased and the cells underwent apoptosis upon TRAIL treatment (Fig. 3B). Infection of normal primary human cervical epithelial cells with either Ad-GFP or Ad-hTERT-DR4 did not result in cell death. The addition of TRAIL to infected cells slightly increased cell death, but less than 10% of the cells underwent apoptosis (Fig. 3C). Taken together, these results suggest that the combination of TRAIL treatment and the infection of an adenovirus expressing TRAIL-death receptor DR4 under the control of the hTERT core promoter has the potential to induce cell death in TRAIL-resistant cancer cells but not in normal cells.
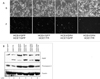
To check the bystander effect of TRAIL produced from the cells infected with Ad-ILZ-TRAIL, TRAIL-resistant HCE4 cells were infected with either Ad-GFP or Ad-hTERT DR4, and TRAIL-resistant HCE7 cells16 were infected with either Ad-GFP or Ad-ILZ-TRAIL. One day after the infection, the cells were harvested and co-cultured for 24 h. When Ad-hTERT DR4 infected HCE4 cells and Ad-ILZ-TRAIL infected HCE7 cells were co-cultured, the induction of apoptosis was evident both morphologically (Fig. 4A) and biochemically (Fig. 4B). These results suggest that DR4-expressing cancer cells might be killed by the bystander effect of TRAIL encoded by the Ad-ILZ-TRAIL vector.
Although TRAIL is a potent inducer of apoptosis in cancer cells, there are still several TRAIL-resistant cancer cells. Many successful approaches have been devised to overcome this resistance, such as the combination of TRAIL with other reagents like DNA damaging agents,14,22 ionizing radiation,23 or virus expressing wild type p53.24 In this study, we tested the effect of combining TRAIL and hTERT core promoter-driven expression of DR4 on the induction of apoptosis in TRAIL-resistant cancer cell lines and normal cells. We found that the hTERT core promoter was able to drive DR4 expression in cancer cells. The combination of infection with a DR4-expressing adenovirus and TRAIL treatment was able induce apoptosis in TRAIL-resistant cancer cells without inducing significant cell death in primary normal cells. In addition, TRAIL expressed from an adenovirus could induce cell death in Ad-hTERT-DR4 infected TRAIL-resistant cancer cells by the bystander effect.
The hTERT promoter was widely used for inducing apoptosis selectively in cancer cells by driving expression of apoptosis-inducing gene products such as bax or TRAIL.25-30 However, the expression of TRAIL alone could only induce apoptosis in TRAIL-sensitive cancer cells and the expression of bax alone could induce apoptosis only in cancer cells that were infected with the adenoviruses. Accordingly, the combined use of the adenovirus that could express TRAIL-death receptor selectively in cancer cells and the adenovirus that could express TRAIL which is then secreted into adjacent cancer cells, thereby exerting the bystander effect, may induce apoptosis in clumps of cancer cells that are resistant to TRAIL. Thus, the generation of an adenovirus that could bicistronically express the death receptor and TRAIL under the control of the hTERT promoter could reduce the number of viral particles used in the experiment, thereby alleviating adenoviral toxicity toward normal cells.
The strategy introduced in this experiment for inducing cancer cell death could be applied to the treatment of cervical cancer because many cervical cancers are associated with the infection of oncogenic HPV, such as types 16 and 18, which can transform normal cells by expressing E6 and E7 oncoproteins.
Figures and Tables
Fig. 1
hTERT core promoter activity in normal cells and cancer cells. The luciferase activity assay was performed using extracts of cells transiently transfected with pGL3-basic, pGL3-control, or pGL3-hTERT vectors for 24 h. pCMV-β-galactosidase was co-transfected in order to standardize the transfection efficiency. The data are expressed as the mean ± SD for triplicate conditions, and similar results were obtained from two independent experiments.

Fig. 2
Expression-, secretion-, and apoptosis-inducing activity of adenoviral-encoded TRAIL. (A) Schematic diagrams of Ad-GFP and Ad-ILZ-TRAIL. (B) Expression and secretion of TRAIL after infection. TRAIL-resistant TE5 cells were infected with either Ad-GFP or Ad-ILZ-TRAIL (Ad-ILZ). Western blot analysis was performed using lysates and culture media 48 h after infection. The arrowhead indicates the un-processed signal peptide containing TRAIL and the arrow represents the fully processed form of TRAIL. (C) Oligomerization of secreted TRAIL. The same media used in (B) was used to confirm the oligomerization of secreted TRAIL. To maintain oligomerization of secreted TRAIL during SDS-PAGE, EGS (final 1 mM) was added into the media. (D) Apoptosis-inducing activities of expressed TRAIL. TRAIL-sensitive SW480 cells were infected with either Ad-GFP (GFP) or Ad-ILZ-TRAIL (TR). After 48 h, the cells were observed under bright field (B) or fluorescent (F) microscopy (× 400). (E) Western blot analysis was performed using the lysates obtained from the same cells as in (D). z-VAD-fmk (20 µM final) was added 1 h after infection.
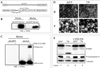
Fig. 3
Cancer cell death induced by the combination of TRAIL treatment and AdhTERT-DR4 infection. (A) Schematic diagram of Ad-hTERT DR4. (B) The expression of DR4 and induction of apoptosis in cancer cell lines in the presence of TRAIL. Western blot analysis was performed using extracts of CasKi (top left) or HCE4 (top right) cells that had been infected with either Ad-GFP or Ad-hTERT DR4 for 24 h and subsequently treated with human recombinant TRAIL for 24 h. Flow cytometric analysis for sub-G1 content. CasKi or HCE4 cells were infected and treated as in (B), and subsequently harvested for flow cytometry. The sub-G1 content of 10,000 cells was examined for each sample (bottom left). This experiment was performed in triplicate. Percent of sub-G1 was plotted (bottom right). (C) Resistance of normal primary cervical keratinocytes. Normal keratinocytes were infected and treated, and cell death was quantified by flow cytometric detection as in (B). This experiment was performed in triplicate. Percent of sub-G1 was plotted.
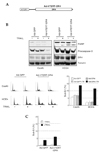
Fig. 4
The bystander effect of adenoviral-encoded TRAIL. (A) Morphologic observation of apoptosis. TRAIL-resistant HCE4 cells were infected with either Ad-GFP (GFP) or Ad-hTERT DR4 (DR4), and TRAIL-resistant HCE7 cells were infected with either Ad-GFP or Ad-ILZ-TRAIL (TR). One day after the infection, the cells were harvested and co-cultured for 24 h. The cells were observed under bright field (B) or fluorescent (F) microscopy (× 100). (B) Biochemical detection of apoptosis. Western blot analysis was performed using the extracts obtained from the cells described in (A).
References
1. Klingelhutz AJ, Foster SA, McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996. 380:79–82.
2. Greider CW, Blackburn EH. Telomeres, telomerase and cancer. Sci Am. 1996. 274:92–97.
3. Nugent CI, Lundblad V. The telomerase reverse transcriptase: components and regulation. Genes Dev. 1998. 12:1073–1085.
4. Park YM, Choi JY, Byun BH, Cho CH, Kim HS, Kim BS. Telomerase is strongly activated in hepatocellular carcinoma but not in chronic hepatitis and cirrhosis. Exp Mol Med. 1998. 30:35–40.
5. Ramakrishnan S, Eppenberger U, Mueller H, Shinkai Y, Narayanan R. Expression profile of the putative catalytic subunit of the telomerase gene. Cancer Res. 1998. 58:622–625.
6. Takakura M, Kyo S, Kanaya T, Tanaka M, Inoue M. Expression of human telomerase subunits and correlation with telomerase activity in cervical cancer. Cancer Res. 1998. 58:1558–1561.
7. Takakura M, Kyo S, Kanaya T, Hirano H, Takeda J, Yutsudo M, et al. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999. 59:551–557.
8. Zhao JQ, Hoare SF, McFarlane R, Muir S, Parkinson EK, Black DM, et al. Cloning and characterization of human and mouse telomerase RNA gene promoter sequences. Oncogene. 1998. 16:1345–1350.
9. French LE, Tschopp J. The TRAIL to selective tumor death. Nat Med. 1999. 5:146–147.
10. Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999. 104:155–162.
11. Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999. 5:157–163.
12. Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997. 276:111–113.
13. Wu GS, Burns TF, McDonald ER 3rd, Jiang W, Meng R, Krantz ID, et al. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet. 1997. 17:141–143.
14. Kim K, Fisher MJ, Xu SQ, El-Deiry WS. Molecular determinants of response to TRAIL in killing of normal and cancer cells. Clin Cancer Res. 2000. 6:335–346.
15. Zhang XD, Franco A, Myers K, Gray C, Nguyen T, Hersey P. Relation of TNF-related apoptosis-inducing ligand (TRAIL) receptor and FLICE-inhibitory protein expression to TRAIL-induced apoptosis of melanoma. Cancer Res. 1999. 59:2747–2753.
16. Kim K, Nakagawa H, Fei P, Rustgi AK, El-Deiry WS. Targeting Bcl-xL in esophageal squamous cancer to sensitize to chemotherapy plus TRAIL-induced apoptosis while normal epithelial cells are protected by blockade of caspase 9. Cell Death Differ. 2004. 11:583–587.
17. Kim SH, Kim K, Kwagh JG, Dicker DT, Herlyn M, Rustgi AK, et al. Death induction by recombinant native TRAIL and its prevention by a caspase 9 inhibitor in primary human esophageal epithelial cells. J Biol Chem. 2004. 279:40044–40052.
18. He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998. 95:2509–2514.
19. El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993. 75:817–825.
20. Harbury PB, Zhang T, Kim PS, Alber T. A switch between two-, three-, and four-stranded coiled coils in GCN4 leucine zipper mutants. Science. 1993. 262:1401–1407.
21. Ozoren N, Kim K, Burns TF, Dicker DT, Moscioni AD, El-Deiry WS. The caspase 9 inhibitor Z-LEHD-FMK protects human liver cells while permitting death of cancer cells exposed to tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2000. 60:6259–6265.
22. Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJ. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factorrelated apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 2000. 60:847–853.
23. Chinnaiyan AM, Prasad U, Shankar S, Hamstra DA, Shanaiah M, Chenevert TL, et al. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci USA. 2000. 97:1754–1759.
24. Kim K, Takimoto R, Dicker DT, Chen Y, Gazitt Y, El-Deiry WS. Enhanced TRAIL sensitivity by p53 over-expression in human cancer but not normal cell lines. Int J Oncol. 2001. 18:241–247.
25. Gu J, Andreeff M, Roth JA, Fang B. hTERT promoter induces tumor-specific Bax gene expression and cell killing in syngenic mouse tumor model and prevents systemic toxicity. Gene Ther. 2002. 9:30–37.
26. Huang X, Lin T, Gu J, Zhang L, Roth JA, Stephens LC, et al. Combined TRAIL and Bax gene therapy prolonged survival in mice with ovarian cancer xenograft. Gene Ther. 2002. 9:1379–1386.
27. Jacob D, Davis J, Zhu H, Zhang L, Teraishi F, Wu S, et al. Suppressing orthotopic pancreatic tumor growth with a fiber-modified adenovector expressing the TRAIL gene from the human telomerase reverse transcriptase promoter. Clin Cancer Res. 2004. 10:3535–3541.
28. Kagawa S, He C, Gu J, Koch P, Rha SJ, Roth JA, et al. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 2001. 61:3330–3338.
29. Lin T, Gu J, Zhang L, Huang X, Stephens LC, Curley SA, et al. Targeted expression of green fluorescent protein/tumor necrosis factor-related apoptosis-inducing ligand fusion protein from human telomerase reverse transcriptase promoter elicits antitumor activity without toxic effects on primary human hepatocytes. Cancer Res. 2002. 62:3620–3625.
30. Lin T, Huang X, Gu J, Zhang L, Roth JA, Xiong M, et al. Long-term tumor-free survival from treatment with the GFP-TRAIL fusion gene expressed from the hTERT promoter in breast cancer cells. Oncogene. 2002. 21:8020–8028.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download