Abstract
This study was designed to examine the relationship between pericardial fluid and plasma CRP levels, and to alterations in other biochemical parameters in patients undergoing Coronary Artery Bypass Grafting (CABG). The study group consisted of 96 Coronary Artery Disease (CAD) patients who were referred to our clinic for a CABG procedure and from whom sufficient amount of pericardial fluid could be collected. The patients were classified into 3 groups: Stable Angina Pectoris (SAP) (n = 27), Unstable Angina Pectoris (USAP) (n = 36), and Post-Myocardial Infarction (PMI) (n = 33). Levels of CRP, glucose, albumin, total protein, Creatine Kinase (CK), Creatine Kinase-MB (CK-MB), and Lactate Dehydrogenase (LDH) were determined in pericardial fluid samples and in simultaneously collected blood samples from radial artery. The pericardial CRP and LDH levels in the PMI group were higher than in the SAP (p = 0.015 and p = 0.000, respectively) and USAP (p = 0.011, p = 0.047) groups. Serum CRP levels in USAP (p = 0.014) and PMI (p = 0.000) groups were higher than those in the SAP group. Pericardial albumin levels in the PMI group were higher than in the USAP group (p = 0.038). In all groups, the pericardial fluid/serum protein ratio was > 0.5, the LDL ratio was > 0.6, and pericardial fluid LDH concentrations were > 300 mg/dl. CRP level of pericardial fluid was significantly higher in the PMI group than in other groups. However, pericardial fluid LDH levels were higher than blood LDH levels in this group and were also higher than pericardial fluid LDH levels of other groups.
Pericardial fluid is located in the space between the parietal and visceral layers of the pericardium and is produced by the visceral pericardium. It has a volume of approximately 15-50 ml and has the function of lubrication.1-3 Since the content of this fluid is similar to that of plasma, it is considered as an ultrafiltrate of plasma and concentrations of many molecules are close to those found in plasma.2-4 Many factors, including systemic diseases, coronary artery diseases, malignant diseases, connective tissue disorders, infections, and idiopathic causes can increase the amount and change the composition of pericardial fluid.1,3-7
Recent studies provide evidence that inflammation plays a role in the pathogenesis of cardiovascular disease. Baseline levels of C-reactive protein (CRP) in apparently healthy persons or patients with stable angina pectoris constitute an independent risk factor for cardiovascular events, whereas the rise in CRP after acute myocardial infarction (AMI) or during unstable angina pectoris (USAP) correlates with outcome. The link between CRP and cardiovascular disease is thought to be indirect in that circulating CRP only reflects the extent of acute phase reaction. Such a reaction can be in response to non-specific stimuli such as confounding risk factors, atherosclerosis, vascular injury, ischemia, and necrosis.8-11 In patients with myocardial infarction, serum CRP levels rise and this increase can persist for more than 4 weeks.9 In patients with coronary heart disease, increased CRP levels might reflect arterial inflammation associated with changes in plaque morphology, rupture, and thrombosis.9
The importance of diagnostic studies in pericardial fluid was emphasized by Myers et al.,5 and Burgess et al.12 drew our attention to the usefulness of biochemical parameters in differential diagnosis of pericardial effusions. The main reason behind the scarcity of studies analyzing pericardial fluid is the difficulty of collecting pericardial fluid samples.
This study was designed to examine the relationship between concentrations of CRP in serum and in pericardial fluid, and other biochemical parameters in patients undergoing coronary artery bypass grafting (CABG).
Out of 145 consecutive CAD patients referred to our clinic for a CABG procedure, 96 patients with a volume of pericardial fluid sample exceeding 10 ml were included in the study. Patients with a sample volume less than 10 ml, and patients on insulin or oral anti-diabetics were excluded (n = 49). Informed consent was obtained from patients and Institutional Ethics Committee approved the study protocol.
Patients were classified into 3 groups: Group 1: Patients with stable angina pectoris (SAP) who were operated on the basis of critical narrowing detected by coronary angiography (n = 27); Group 2: Patients with a diagnosis of unstable angina pectoris (USAP) (n = 36); and Group 3: Patients suffering from recent (< 4w) MI (post- myocardial infarct = PMI) (n = 33).
CRP, glucose, albumin, total protein, Creatine Kinase (CK), Creatine Kinase-MB (CK-MB) and Lactate Dehydrogenase (LDH) levels were determined in pericardial fluid samples and in simultaneously collected blood samples from radial artery. In order to estimate the percentage of CK-MB in total CK, percent relative index (PRI) was used.
CRP levels were determined by turbidimetric tests (Boehringer Mannheim, the measurement range is 0.3 to 24 mg/dl, with coefficients of variation within assays ranging from 0.6% to 1.3% and between-assays ranging from 1.3% to 6.0% at different levels of CRP); glucose and protein were measured by ion-selective methods; and albumin, LDH, CK and CK-MB were measured by spectrophotometric methods.
The results were expressed as mean ± standard deviation. All analyses were performed using SPSS software for windows (SPSS Inc, Chigaco, IL, USA) and differences were considered statistically significant at a probability level of less than 0.05.
Results in the three groups were compared with repeated measured analysis of variance (ANOVA) followed by Bonferroni post-hoc test.
There were no significant differences between groups with regard to age, gender, and BMI (Table 1). Ejection fraction (EF) was significantly lower in the PMI group than in the USAP group (p = 0.007).
Results of biochemical analyses in pericardial fluid and arterial samples are shown in Table 2.
The pericardial fluid CRP and serum LDH levels were significantly higher in the PMI group than in SAP (p = 0.015, p = 0.000) and USAP (p = 0.011, p = 0.047) groups. Pericardial fluid albumin concentration was higher in the PMI group than in the USAP group (p = 0.038). Serum CRP levels in the SAP group were significantly lower than those in USAP (p = 0.014) and in PMI (p = 0.000) groups.
CK-MB PRI levels in pericardial fluid (SAP = 21, USAP = 40 and PMI = 34) were higher than in serum (SAP = 8, USAP = 8 and PMI = 7). While the ratios in serum were similar across the study arms, the pericardial fluid levels were remarkably high in USAP and PMI groups.
The serum protein and LDH ratios for pericardial fluid are depicted in Fig. 1. In all groups, pericardial fluid/serum protein ratios were larger than 0.5, and pericardial fluid/serum LDH ratios were larger than 0.6. Furthermore, the LDH concentrations in pericardial fluid were above 300 mg/dl in all groups (Table 2). These results demonstrate that the pericardial fluid in all three arms of the study has the characteristics of an exudate based on Light's criteria.
In this study serum CRP levels were significantly higher in USAP and PMI groups than in the SAP group. However, we detected high pericardial fluid CRP levels only in the PMI group. These findings underscore the importance of epicardial diffusion, in which only molecules smaller than 40 kilo Daltons (kD) can readily diffuse into pericardial fluid. Larger molecules can not enter.2,4 Human CRP cannot enter the pericardial cavity by epicardial diffusion mechanisms because of its size (118 kD) and pentameric structure. However in conditions like AMI or agonal myocarditis, inflammatory changes such as local vasodilation and increased permeability make CRP molecules easily diffuse into pericardial cavity.13 Since there is no local production of CRP in pericardial cavity, CRP molecules discovered in pericardial fluid suggest that the epicardial diffusion mechanism is altered or enhanced by local inflammatory reactions in AMI patients. CRP levels in USAP patients were high in serum, but low in pericardial fluid. This finding suggests that the inflammatory reactions are not intense enough to alter or enhance the epicardial diffusion mechanism in USAP patients. During ischemia-reperfusion, cellular membranes are particularly susceptible to oxygen radical damage because of their high content of polyunsaturated fatty acids. Lipid peroxidation is among the main effects resulting from free radical release in biological membranes. Lipid peroxidation is initiated by cell damage.14-16 Cellular and tissue edema resulting from the disruption of membrane permeability during ischemia-reperfusion unfavorably affected epicardial diffusion in the PMI group. However epicardial diffusion seems to be unaffected in USAP and SAP groups We observed no ischemiareperfusion event resulting in cellular damage.
The distinction between exudate and transudate is usually based on Light's criteria. A fluid/serum protein ratio greater than 0.5, a fluid/serum lactate dehydrogenase (LDH) ratio greater than 0.6, and a fluid LDH concentration greater than 300 U/L is indicative of exudate. If the total protein content in the fluid is greater than 3.0 g/dL the sensitivity is 97%, and if the fluid/serum protein ratio is greater than 0.5 the sensitivity is 96%. A LDH level greater than 300 mg/dl is 98% sensitive for exudate; and levels of fluid/serum LDH greater than 0.6 is 94% sensitive for exudate.3,5,17
In all 3 groups of patients in our study, the pericardial fluid samples had the characteristics of an exudate based on Light's criteria. The high LDH levels and fluid/serum LDH ratios in USAP and PMI groups were remarkable. We believe that pericardial fluid should be evaluated in a different way than other body fluids while examining fluid LDH levels, the most sensitive marker for transudate-exudate distinction based on Light's criteria. This is because of the probability of an increased diffusion of LDH into pericardial fluid due to an abnormal epicardial permeability that increases the total LDH level, A study of subgroups of LDH enzymes in pericardial fluid would shed more light on this subject.
Meyers et al.5 report that glucose levels are higher when the pericardial fluid has the characteristics of an exudate, and that the pericardial fluid/serum glucose ratio is > 1 when the fluid is transudate and < 1 when it is exudate. In our study, glucose levels in pericardial fluids in all groups were lower than those in serum .
Spodick1 reports that the protein content of pericardial fluid is lower than that of plasma, but albumin levels in pericardial fluid may be higher than in plasma. This is owing to albumin's lower molecular weight and ease of transport. In our study, serum levels of albumin and protein were higher than pericardial fluid levels in all groups. This finding is in line with previously reported results.1,5
Creatine kinase is composed of 2 subunits, each with a molecular weight of 43 kD.18,19 In our study, concentrations of CK in pericardial fluid were lower than those in serum whereas the CK-MB levels in pericardial fluid were higher. CK-MB PRI in serum was similar across the three groups, but the CK-MB PRI in pericardial fluid was significantly higher in USAP and PMI groups. These high levels in USAP and PMI groups are indirect findings indicating increased release of this enzyme from myocytes.
In conclusion, pericardial fluid CRP levels in the PMI group were greater than in other groups. Pericardial fluid LDH levels were also higher than blood LDH levels in the PMI group, and higher than pericardial LDH levels of other groups. Although these results do not provide a new treatment approach, they are important as they are the first CRP analysis results of pericardial fluid in living humans.
Figures and Tables
Fig. 1
Results of pericardial fluid analysis according to Light's criteria. SAP, Stable Angina Pectoris; USAP, Unstable Angina Pectoris; PMI, Post-Myocardial Infarction.
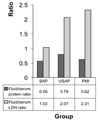
Table 2
Results of Pericardial Fluid and Simultaneous Blood Samples

*vs. SAP, p = 0.015; †vs. USAP, p = 0.011; ‡vs. SAP, p = 0.000; §vs. USAP, p = 0.047; ∥vs. USAP, p = 0.038; **vs. USAP, p = 0.014; ††vs. PMI, p = 0.000 (One way ANOVA test).
SAP, Stable Angina Pectoris; USAP, Unstable Angina Pectoris; PMI, Post-Myocardial Infarction; PF, Pericardial fluid; CRP, C-Reactive Protein; CK, Creatine Kinase; CK-MB, Creatine Kinase-MB; LDH, Lactate Dehydrogenase.
References
1. Spodick DH. Diagnostic interpretation of pericardial fluids. Chest. 1997. 111:1156–1157.
2. Oyama J, Shimokawa H, Morita S, Yasui H, Takeshita A. Elevated interleukin-1β in pericardial fluid of patients with ischemic heart disease. Coron Artery Dis. 2001. 12:567–571.
3. Strimel WJ, Noe S. Pericardial effusion. eMedicine Journal. 2002. 3.
4. Corda S, Mebazaa A, Gandolfini MP, Fitting C, Marotte F, Peynet J, et al. Trophic effect of human pericardial fluid on adult cardiac myocytes. Differential role of fibroblast growth factor-2 and factors related to ventricular hypertrophy. Circ Res. 1997. 81:679–687.
5. Meyers DG, Meyers RE, Prendergast TW. The usefulness of diagnostic tests on pericardial fluid. Chest. 1997. 111:1213–1221.
6. Atar S, Chiu J, Forrester JS, Siegel RJ. Bloody pericardial effusion in patients with cardiac tamponade: is the cause cancerous, tuberculous, or iatrogenic in the 1990s? Chest. 1999. 116:1564–1569.
7. Obaji A, Efferen LS. Diagnostic tests on pericardial fluid. Chest. 1998. 114:345.
8. Lagrand WK, Visser CA, Hermens WT, Niessen HW, Verheugt FW, Wolbink GJ, et al. C-reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation. 1999. 100:96–102.
9. Fransen EJ, Maessen JG, Elenbaas TW, van Aarnhem EE, van Dieijen-Visser MP. Enhanced preoperative C-reactive protein plasma levels as a risk factor for post-operative infections after cardiac surgery. Ann Thorac Surg. 1999. 67:134–138.
10. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000. 102:2165–2168.
11. Fichtlscherer S, Rosenberger G, Walter DH, Breuer S, Dimmeler S, Zeiher AM. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation. 2000. 102:1000–1006.
12. Burgess LJ, Reuter H, Taljaard JJ, Doubell AF. Role of biochemical tests in the diagnosis of large pericardial effusions. Chest. 2002. 121:495–499.
13. Laurier E, Gosset D, Hennache B, Nuttens MC, Debuire B, Lenoir L, et al. Pericardial C-reactive protein. A marker of agonal cardiac disease? Presse Med. 1991. 20:405–408.
14. Maulik N, Yoshida T, Das DK. Oxidative stress developed during the reperfusion of ischemic myocardium induces apoptosis. Free Radic Biol Med. 1998. 24:869–875.
15. Park JL, Lucchesi BR. Mechanism of myocardial reperfusion injury. Ann Thorac Surg. 1999. 68:1905–1912.
16. Piper HM, Garcia-Dorado D. Prime causes of rapid cardiomyocyte death during reperfusion. Ann Thorac Surg. 1999. 68:1913–1919.
17. Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of pleurodesis failure: analysis of primary data. Chest. 2000. 117:87–95.
18. Wu AH, Apple FS, Gibler WB, Jesse RL, Warshaw MM, Valdes R Jr. National Academy of Clinical Biochemistry Standards of Laboratory Practice: recommendations for the use of cardiac markers in coronary artery diseases. Clin Chem. 1999. 45:1104–1121.
19. Couthon F, Clottes E, Vial C. High salt concentrations induce dissociation of dimeric rabbit muscle creatine kinase. Physico-chemical characterization of the monomeric species. Biochim Biophys Acta. 1997. 1339:277–288.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


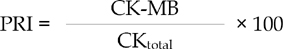

 XML Download
XML Download