Abstract
We investigated the expression of membrane type-1 (MT1)-MMP, MMP2, MMP9 and TIMP2 mRNAs and their roles in ductal carcinoma in situ (DCIS) and T1 and T2 invasive ductal carcinoma of the breast. We further compared these two types of carcinomas for differences in microvessel density, and expression of angiogenic factors and CD44std. MT1-MMP, MMP2, MMP9 and TIMP2 mRNA were expressed in both DCIS and invasive ductal carcinomas. Expression rates of MT1-MMP, MMP2, MMP9 and TIMP2 mRNAs were not statistically different between DCIS and invasive ductal carcinomas, nor did they differ statistically when grouped by tumor size, histologic grade or nuclear grade of invasive ductal carcinoma. Microvessel density and expression of VEGF and TGF-β1 were not statistically different between DCIS and invasive ductal carcinoma. CD44std expression was significantly increased in DCIS compared to invasive ductal carcinoma (p < 0.05) and it was also significantly increased in lower clinical stage, histologic grade and nuclear grade of invasive ductal carcinoma (p < 0.05). Axillary node metastasis was significantly correlated with MT1-MMP mRNA, VEGF and TGF-β1 expression (p < 0.05) and MT1-MMP mRNA was positively correlated with VEGF expression and TIMP2 mRNA (p < 0.05). In summary, patterns of MMP mRNA expression in DCIS and invasive ductal carcinoma suggest that the invasive potential of breast carcinoma is already achieved before morphologically overt invasive growth is observed. As MT1-MMP mRNA expression is significantly correlated with axillary nodal metastasis, it may be useful as a prognostic indicator of invasive ductal carcinoma. Considering the positive correlation of MT1-MMP mRNA and TIMP2mRNA expression, our finding supports a role for TIMP2 in tumor growth, as well as the utility of CD44std as a prognostic indicator of breast cancer.
Go to : 
Degradation of basement membranes and extracellular matrix is an essential process in invasion and metastasis in malignant tumors. MMP, potent proteolytic enzymes are known to play key roles in this process. Within the MMP families, MMP-2 (gelatinase A, 72 kDa) and MMP-9 (gelatinase B, 92 kDa) cleave the type IV collagen and gelatin, which are the principal structural components of basement membrane.1 Expression of MMP2 and MMP9 has been reported in breast cancer,2-4 colon cancer,5 skin tumors6 and lung cancer7 and their expression has been correlated with local invasion by the tumor, lymph node metastasis and survival rates.2,3 Membrane type-1 (MT1) MMP specifically activates the pro-gelatinase, MMP2 on the tumor cell surface in vitro and binds with TIMPs, hence, may have a role in controlling MMP2 activity.8 The cell surface adhesion molecule, CD44, is also an important factor in invasion and metastasis.9-11 A recent report showed that CD44 can localize active MMP9 protein to the tumor cell surface where the CD44-MMP9 complex induces TGF-β1 activation, which in turn promotes tumor invasion and angiogenesis.12
To further understand their roles in breast cancer, we investigated the expression of MT1-MMP, MMP2, MMP9 and TIMP2 mRNAs using mRNA in situ hybridization and microvessel density counts. The expression of angiogenic factors and CD44std as measured by immunohistochemical staining was compared in ductal carcinoma in situ (DCIS) and T1 and T2 invasive ductal carcinoma of the breast. Further, we explored the roles of MT1-MMP, MMP2, MMP9 and TIMP2 and the relationship between MMP and TMP mRNA, CD44std, microvessel density and angiogenic factors.
Go to : 
Primary breast cancer tissue obtained from 84 mastectomy patients was examined. The samples included 21 cases of DCIS and 63 cases of T1 or T2 invasive ductal carcinoma with the tumor size of less than 5 cm. Patients with multiple foci of disseminated tumor and a history of previous axillary surgery on the same side of the body were excluded.
The patients' age, tumor size, lymph node status, clinical stage and histologic findings were reviewed. Nuclear grades of DCIS and invasive ductal carcinoma were grouped according to Black's criteria.13 Histologic grading of invasive ductal carcinoma was classified according to the modified Bloom and Richardson method.14
Immunohistochemical staining was performed by the labeled streptavidin-biotin method using a LSAB kit (DAKO, Carpinteria, CA, USA). One representative section of the tissue was cut at 4 µm and placed on poly-L-lysine coated slides. The slides were deparaffinized, rehydrated, immersed in 10 mM sodium citrate (pH 6.0), and pretreated in a microwave oven for 10 minutes, followed by rinsing with TBS buffer (pH 7.6) for 10 minutes. After blocking with 3% hydrogen peroxide and normal goat serum, respectively, for 30 minutes each, the slides were incubated at 4℃ overnight with primary antibodies. The primary antibodies employed were as follows: anti-CD44std (H-CAM, mouse monoclonal antibody, Novocastra, Newcastle, UK, 1:50), anti-Factor VIII (clone F8/86, mouse monoclonal antibody, DAKO, CA, USA, 1:50), anti-TGF-β1 (TB21, mouse antihuman antibody, Serotec, Oxford, UK, 1:100), and anti-VEGF (sc-152, rabbit polyclonal antibody, Santa Cruz, CA, USA, 1:250). After incubation with the primary antibody, the slides were treated with, biotinylated anti-mouse, rabbit IgG (DAKO) and a complex of peroxidase conjugated streptavidin (DAKO) in the order listed. The final reaction products were visualized with 3-amino-9-ethylcarbazole and light hematoxylin counterstain.
Immunohistochemical staining for CD44std was interpreted as positive when more than 10% of tumor cells were stained along the cytoplasmic membrane. A slide was scored as positive for TGF-β1 and VEGF when more than 5% of tumor cells were stained in the cytoplasm of tumor cells.
One representative tumor block was stained with anti-Factor VIII antibody. Singular or clusters of endothelial cells irrespective of whether they formed a lumen were counted as individual microvessels and blood vessels with thick muscular walls were excluded. Microvessels were counted in the highly vascular field of the tumor-stroma interface or the central area of the tumor at × 200 magnification and the average number of microvessels obtained in three different fields was calculated.
In situ hybridization was done with the Inno GenexTM universal ISH detection kit (InnoGenex, San Ramon, CA, USA). The following probes were labelled with FITC: MT1-MMP, MMP9, TIMP2 (human, Biognostik, Germany) DNAs and and a MMP2 oligonucleotide (5'-TGG/GCT/ACG/GCG/CGG/CGG/CGT/GGC-3'FITC, Genemed Synthesis, Inc., South San Francisco, CA, USA.) Deparaffinized sections were blocked by washing in sterile deionized water containing 0.2% RNase for 5 minutes and pretreated with proteinase K at 3 7℃ for 15 minutes. Slides were heated in a microwave for 2 minutes, cooled at room temperature for 10 minutes, postfixed with 1% formalin/RNase-free PBS for 10 minutes, and washed twice with sterile deionized water containing 0.2% RNase. Probes (MMP2 1:20, MMP9 1:15, TIMP2 1:15, MT1-MMP 1:15) were diluted into the manufacturer's hybridization solution and applied to the slides. A coverglass was applied and slides were heated to 80℃ for 5 minutes before hybridizing at 37℃ for 16 hours. Posthybridization washing was done in 1 × and 0.1 × SSC solution containing power block reagent for 5 minutes at room temperature followed by biotinylated anti-probe antibodies for 20 minutes at room temperature. After washing with 1 × PBS, slides were treated with streptavidin horseradish peroxidase conjugate for 20 minutes, stained with aminoethyl carbazole (AEC), and examined by light microscopy.
Positive controls were made with fluoresceinated poly (A) oligonucleotides and the negative controls made with hybridization solution only without probe. Determination of a positive reaction was made when the nuclear and/or cytoplasmic staining of cells was more densely stained than background.
Statistical analysis was performed using the SPSS version 9.0 software program and a statistical difference was considered significant when the p value was 0.05 or less.
Go to : 
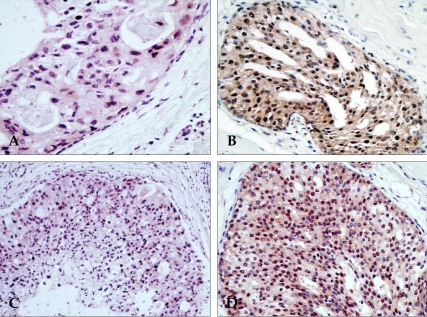
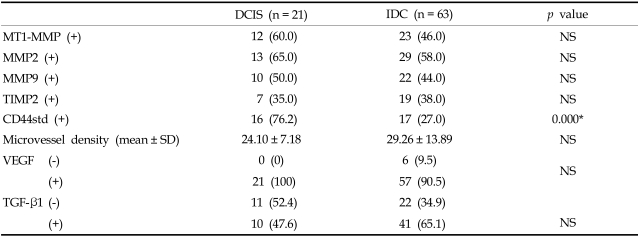
MT1-MMP, MMP2, MMP9 and TIMP2 mRNAs were expressed in 60%, 65%, 50%, and 35% of DCIS and 46%, 58%, 44% and 38% of invasive ductal carcinoma, respectively (Table 1). There was no statistical difference in MMP and TIMP mRNA expression levels between DCIS and invasive ductal carcinoma. MMP mRNA expression using in situ hybridization was more intense at the peripheral rim of tumor nests of the invasive ductal carcinoma. Tumor cells were stained in both nuclei and cytoplasm with the nuclei exhibiting a stronger signal. Stromal cells were occasionally stained, but less intensely than in tumor cells (Fig. 1 and 2). A few normal ductal cells were weakly stained.
Microvessel density within the tumor was 24.1 ± 7.18 in DICS and 29.26 ± 13.89 in invasive ductal carcinoma (Table 1). The microvessel density tended to increase in invasive ductal carcinoma compared to DCIS. The distribution of microvessel in DCIS was conspicuous around the basement membrane of tumor nests, which formed periductal cuffing. In contrast, microvessels were usually distributed in the peripheral rather than central zone of the tumor mass in invasive ductal carcinoma. Expression of VEGF and TGF-β1 was not statistically different between DCIS and invasive ductal carcinoma.
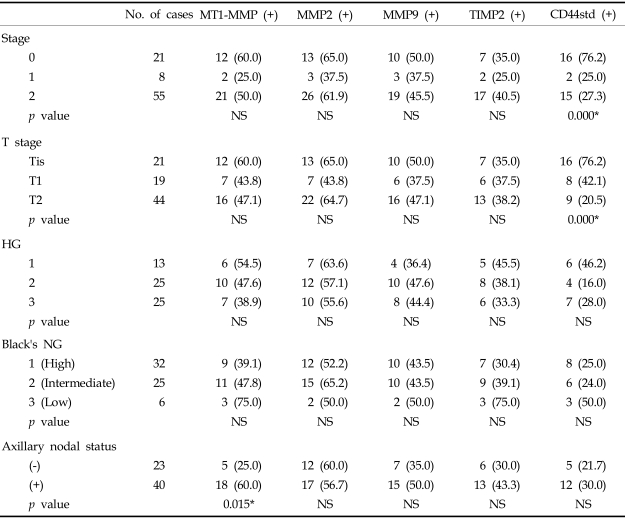
Expression levels of MT1-MMP, MMP2, MMP9 and TIMP2 mRNA did not significantly differ with clinical stage, tumor size, histologic grade or nuclear grade of invasive ductal carcinoma. MT1-MMP, MMP9 and TIMP2 mRNA were expressed at higher levels in cases with axillary nodal metastasis than those without axillary nodal metastasis, with MT1-MMP mRNA expression significantly correlated with axillary nodal metastasis (p < 0.05)(Table 2).
CD44std expression was significantly increased in low clinical stage and small tumors (p < 0.05) and was increased, but not significantly, in low histologic and nuclear grade tumors. CD44std expression was not significantly correlated with axillary nodal status (Table 2).
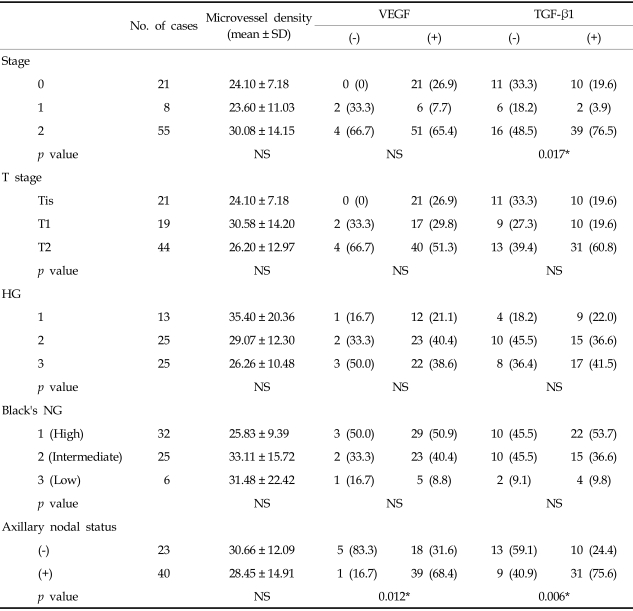
Microvessel densities were not statistically different between clinical stages: 24.10 ± 7.18 in stage 0, 23.60 ± 12.97 in stage 1 and 30.08 ± 14.15 in stage 2 (Table 3.) Microvessel density did not increase significantly with tumor size (24.10 ± 7.18 in Tis, 26.20 ± 12.97 in T1 and 30.58 ± 14.20 in T2.) Microvessel density showed no significant difference with the histologic grade, nuclear grade and axillary nodal status of invasive ductal carcinoma.
TGF-β1 expression varied significantly with the clinical stage (p < 0.05)(Table 3). However, TGF-β1 expression did not differ significantly with tumor size, histologic grade and nuclear grade of invasive ductal carcinoma, On the other hand, the rate of TGF-β1 expression was increased in the high histologic and nuclear grades.
VEGF expression did not vary significantly with the clinical stage, tumor size, histologic grade or nuclear grade of invasive ductal carcinoma (Table 3). However, expression of VEGF and TGF-β1 was significantly higher in cases with axillary nodal metastasis compared to those without axillary nodal metastasis (p < 0.05). Expression of VEGF and TGF-β1 showed no significant correlation with microvessel density.
MT1-MMP, MMP2, MMP9 and TIMP2 mRNA levels were not significantly correlated with microvessel density, TGF-β1, or CD44std. Among the MMPs, MT1-MMP mRNA was positively correlated with VEGF and TIMP2 mRNA (p < 0.05) (Table 4).
Go to : 
This study was performed to demonstrate the expression of MMP and TIMPs mRNA and to determine their roles in noninvasive and invasive ductal carcinoma of the breast. MMP and TIMP mRNA expression in invasive mammary carcinoma has been extensively studied. However, MMP and TIMP mRNA expression levels in noninvasive mammary carcinoma have not been closely examined to date.
We compared the expression of MT1-MMP, MMP2, MMP9 and TIMP2 mRNAs of DCIS with that of invasive ductal carcinoma of breast. MT1-MMP, MMP2, MMP9 and TIMP2 mRNAs were expressed in both DCIS and invasive ductal carcinoma. Moreover, their expression rate was not significantly increased in invasive ductal carcinoma compared to DCIS. Brummer, et al.15 reported MMP and TIMP mRNA expression using in situ hybridization in benign and malignant breast disease. In their study, normal breast and fibrocystic disease showed rare MMP1 and MMP2 mRNA expression and noninvasive ductal carcinomas showed elevated MMP2 transcript levels in peritumoral stromal cells without significant MMP1 signals. Invasive ductal carcinoma displayed co-expression of MMP1 and MMP2 mRNA in peritumoral stromal cells, mainly at the invasion front. The MMP2 signal appeared to increase gradually as the carcinomas progressed from noninvasive to invasive states. Brummer's15 and our results of MMP mRNA expression in noninvasive ductal carcinoma may suggest that an invasive potential of breast carcinoma is already obtained before morphologically overt invasive growth is observed. We believe that further study is war ranted to determine MMP mRNA expression levels according to histologic grades of DCIS in a large population.
Our study using in situ hybridization showed that MMP/TIMP mRNA expression was mainly noted in tumor cells rather than in stromal cells and displayed strong intensity at the interface between tumor cell nests and the interstitial stroma. Variation in the localization of MMPs has been reported depending on the laboratory methods or specific organ types. In breast tissue, MMP expression using ELISA, northern blotting, zymography and in situ hybridization has primarily been observed in peritumoral interstitial fibroblasts and not in tumor cells,16-19 In contrast, MMP protein expression using immunohistochemical staining demonstrated that levels were higher in tumor cells than in interstitial fibroblasts.2,3,20,21 However, MMP mRNA and protein levels in transitional carcinoma of the upper urinary tract and MT1-mRNA in cervical carcinoma and squamous carcinoma of lung were greater in tumor cells than in stromal cells.22-24 It is not clear why these discrepancies are observed; variations in laboratory methods or differences in organs likely play a role. Regardless, these results suggest a close relationship between tumor cells and interstitial cells through a paracrine effect.
Our study showed no significant difference of MT1-MMP, MMP2, MMP9 and TIMP2 mRNA expression levels with stage, tumor size, histologic grade or nuclear grade of invasive ductal carcinoma. Only MT1-MMP mRNA expression was significantly correlated with axillary nodal metastasis. These results concur with Ueno's study25 that MT1-MMP mRNA expression was significantly correlated with lymph node metastasis and distant metastasis and were not significantly correlated with histologic grade. In our study, MT1-MMP mRNA expression was not significantly different between DCIS and invasive ductal carcinoma. However, MT1-MMP mRNA expression was significantly correlated with axillary nodal metastasis. From the above results, MT1-MMP mRNA is considered to involve distant metastasis rather than direct invasion of tumor.
MMPs are balanced with TIMPs, involved in inhibition of MMP and regulation of their activation in vitro. The delicate balance between MMP and TIMP levels ultimately determines the functions of MMP by protein degradation in vitro.26 Most of the MMP expression induced by activated MMP accompanies increased expression of one more TIMPs as a defense mechanism. TIMP2 protein inhibits the activation of proMMP2 by forming a stable complex with activated MT1-MMP, whereas low concentrations of TIMP2 can form a tertiary complex with MT1-MMP and proMMP2 to eventually activate MMP2. Thus, MT1-MMP combines with TIMP2 and influences the activation state of MMP2. Earlier studies reported that TIMPs inhibit tumor growth and metastasis, but recent studies demonstrated that TIMPs actually enhance tumor growth as well as inhibit MMP. Adding to the controversy, TIMP expression and the imbalance of MMP/TIMP have been mentioned in recent studies to be poor prognostic factors27,28 or as important factors in the tumor progression. Our study demonstrated that MT1-MMP mRNA expression is positively correlated with TIMP2 mRNA (p < 0.05) and that a correlation between MT1-MMP mRNA and MMP2 mRNA is not found. Considering the positive correlation of MT1-MMP mRNA and TIMP 2mRNA expression, our finding supports a role for TIMP2 in tumor growth.
The cell surface adhesion molecule CD44 is an important factor in invasion and metastasis. However, the role of CD44 as a prognostic indicator for breast carcinoma has been controvertial. Lyzak, et al.10 demonstrated that CD44std expression is negatively correlated with lymph node metastasis and CD44v expression is not significantly correlated with lymph node metastasis in non-palpable T1a and T1b breast cancer. In contrast, some reports11,29 showed CD44std expression correlated with good prognosis and CD44v6 expression correlated with a poor prognostic outcome, especially in low grade Hodgkin's lymphoma, cervical carcinoma of stage I and IIB, ovarian cancer and rectal cancer.30-33 Our study showed that CD44std expression was significantly higher in DCIS (76.2%) than in invasive ductal carcinoma (27%) and significantly higher in low histologic (46.2%) and nuclear (50.0%) grade than in high histologic (28.0%) and nuclear (25.0%) grade invasive ductal carcinoma. The above results suggest that CD44std could be used as a prognostic indicator of breast cancer. Yu et al.12 recently showed that CD44 can localize active MMP9 to the tumor cell surface with the CD44-MMP9 complex providing TGF-β1 activation to promote tumor invasion and angiogenesis. We failed to find a connection between CD44std, MMP9 and TGF-β1.
The intratumoral microvessel density was not statistically different in invasive ductal carcinoma and DCIS. However, microvessel density tended to increase in cases with increased expression of VEGF. In DCIS, the stromal microvessels actively proliferated around the basement membrane of tumor cell nests forming periductal cuffing. In contrast, microvessels were prominent in the peripheral tumor margin revealing more invasive growth peripherally than in the center of the tumor in invasive ductal carcinoma. We conclude from these findings that invasive growth is indirectly correlated with microvessel proliferation.
In summary, MT1-MMP, MMP2, MMP9 and TIMP mRNAs, which are involved in stromal invasion, are expressed in both DCIS and invasive ductal carcinoma at similar levels. The MMP mRNA expression levels suggest that an invasive potential of breast carcinoma is already obtained before morphologically overt invasive growth is observed. As MT1-MMP mRNA expression is significantly correlated with axillary nodal metastasis, it might be considered as a significant prognostic indicator for invasive ductal carcinoma. Considering the positive correlation between MT1-MMP mRNA and TIMP2 mRNA expression, our findings support a role for TIMP2 in tumor growth. Further, CD44std could be used as a prognostic indicator of breast cancer. As our study is limited by the small number of cases, a larger study is warranted to confirm the prognostic value of MMP/TIMPs in breast cancer and precancerous lesions.
Go to : 
Notes
This study was supported in part by the Yonsei University Research Fund of 2001 and by the BMS Korea Research Fund of 2001.
Go to : 
References
1. Toi M, Ishigaki S, Tominaga T. Metalloproteinases and tissue inhibitors of metalloproteinases. Breast Cancer Res Treat. 1998; 52:113–124. PMID: 10066076.

2. Talvensaari-Mattila A, Pääkkö P, Höyhtyä M, Blanco-Sequeiros G, Turpeenniemi-Hujanen T. Matrix metalloproteinase-2 immunoreactive protein: a marker of aggressiveness in breast carcinoma. Cancer. 1998; 83:1153–1162. PMID: 9740080.
3. Talvensaari-Mattila A, Pääkkö P, Blanco-Sequeiro G, Turpeenniemi-Hujanen T. Matrix metalloproteinase-2 (MMP2) is associated with the risk for a relapse in postmenopausal patients with node-positive breast carcinoma treated with antiestrogen adjuvant therapy. Breast Cancer Res Treat. 2001; 65:55–61. PMID: 11245340.

4. Scorilas A, Karameris A, Arnogiannaki N, Ardavanis A, Bassilopoulos P, Trangas T, et al. Overexpression of matrix-metalloproteinase-9 in human breast cancer: a potential favorable indicator in node-negative patients. Br J Cancer. 2001; 84:1488–1496. PMID: 11384099.
5. Liabakk NB, Talbot I, Smith RA, Wilkinson K, Ballewill F. Matrix metalloproteinase 2 (MMP-2) and matrix metalloproteinase 9 (MMP-9) type IV collagenase in colorectal cancer. Cancer Res. 1996; 56:190–196. PMID: 8548762.
6. Pyke C, Ralfkier E, Huhtala P, Hurskainen T, Dano K, Tryggvason K. Localization of messenger RNA for Mr 72,000 and 92,000 type IV collagenase in human skin cancers by in situ hybridization. Cancer Res. 1992; 52:1336–1341. PMID: 1310643.
7. Kodate M, Kasai T, Hashimoto H, Yabumoto K, Iwata Y, Manobe H. Expression of matrix metalloproteinase (gelatinase) in T1 adenocarcinoma of the lung. Pathol Int. 1997; 47:461–469. PMID: 9234385.

8. Sato H, Takano T, Kinoshita T, Imai K, Okada Y, Stetler-Stevenson WG, et al. Cell surface binding and activation of gelatinase A induced by expression of membrane-type-1-matrix metalloproteinase (MT1-MMP). FEBS Lett. 1996; 385:238–240. PMID: 8647259.

9. Schneider J, Pollan M, Ruibal A, Jimenez E, Lucas AR, Nunez MI, et al. Histologic grade and CD44 are independent predictors of axillary lymph node invasion in early (T1) breast cancer. Tumour Biol. 1999; 20:319–330. PMID: 10567878.

10. Lyzak JS, Yaremko ML, Recant W, Baunoch DA, Joseph L. Role of CD44 in nonpalpable T1a and T1b breast cancer. Hum Pathol. 1997; 28:772–778. PMID: 9224743.

11. Joensuu H, Klemi PJ, Toikkanen S, Jalkanen S. Glycoprotein CD44 expression and its association with survival in breast cancer. Am J Pathol. 1993; 143:867–874. PMID: 8362982.
12. Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000; 14:163–176. PMID: 10652271.
13. Black MM, Speer FD. Nuclear structure in cancer tissues. Surg Gynecol Obstet. 1957; 105:97–102. PMID: 13442910.
14. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. The value of histologic grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991; 19:403–410. PMID: 1757079.
15. Brummer O, Athar S, Riethdorf L, Loning T, Herbst H. Matrix-metalloproteinases 1,2, and 3 and their tissue inhibitors 1 and 2 in benign and malignant breast lesions: an in situ hybridization study. Virchows Arch. 1999; 435:566–573. PMID: 10628798.
16. Nielsen BS, Schested M, Kjeldsen L, Borregaard N, Rygaard J, Danon K. Expression of matrix metalloproteinase-9 in vascular pericytes in human breast cancer. Lab Invest. 1997; 77:345–355. PMID: 9354769.
17. Duffy MJ, Blaser J, Duggan C, McDermott E, O'Higgins N, Fennelly JJ, et al. Assay of matrix metalloproteinase types 8 and 9 by ELISA in human breast cancer. Br J Cancer. 1995; 71:1025–1028. PMID: 7734294.
18. Pacheo MM, Mourao M, Mantovani EB, Nishimoto IN, Brentani MM. Expression of gelatinase A and B, stromelysin-3 and matrilysin genes in breast carcinomas: clinico-pathological correlations. Clin Exp Metastasis. 1998; 16:577–585. PMID: 9932604.
19. Rha SY, Yang WI, Kim JH, Roh JK, Min JS, Lee KS, et al. Different expression patterns of MMP-2 and MMP-9 in breast cancer. Oncol Rep. 1998; 5:875–879. PMID: 9625836.

20. Ishigaki S, Toi M, Ueno T, Matsumoto H, Muta M, Koike M, et al. Significance of membrane-type-1-matrix metalloproteinase expression in breast cancer. Jpn J Cancer Res. 1999; 90:516–522. PMID: 10391091.
21. Jones JL, Glynn P, Walker RA. Expression of MMP-2 and MMP-9, their inhibitors and the activator MT1-MMP in primary breast carcinoma. J Pathol. 1999; 189:161–168. PMID: 10547569.
22. Nakanishi K, Kawai T, Sato H, Aida S, Kasamatsu H, Aurues T, et al. Expression of matrix metalloproteinase-2 (MMP-2) and of membrane-type-1-matrix metalloproteinase (MT1-MMP) in transitional cell carcinoma of the upper urinary tract. Hum Pathol. 2000; 31:193–200. PMID: 10685633.
23. Gilles C, Polette M, Piette J, Munaut C, Thompson EW, Birembaut P, et al. High level of MT-MMP expression is associated with invasiveness of cervical cancer cells. Int J Cancer. 1996; 65:209–213. PMID: 8567119.

24. Polette M, Nawrocki B, Gilles C, Sato H, Seiki M, Tournier JM, et al. MT-MMP expression and localization in human lung and breast cancers. Virchows Arch. 1996; 428:29–35. PMID: 8646366.
25. Ueno H, Nakamura H, Inoue M, Imai K, Noguchi M, Sato H, et al. Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in human invasive breast carcinomas. Cancer Res. 1997; 57:2055–2060. PMID: 9158005.
26. Liotta LA, Stetler-Steverson WG. Tumor invasion and metastasis. an imbalance of positive and negative regulation. Cancer Res. 1991; 51:5054s–5059s. PMID: 1884381.
27. Curran S, Murray GI. Matrix metalloproteinase in tumour invasion and metastasis. J Pathol. 1999; 189:300–308. PMID: 10547590.
28. Massi D, Franchi A, Ketabchi S, Paglierani M, Pimpinelli N, Santucci M. Expression and prognostic significance of matrix metalloproteinases and their tissue inhibitors in primary neuroendocrine carcinoma of the skin. Hum Pathol. 2003; 34:80–88. PMID: 12605370.

29. Kaufmann M, Heider K, Sinn HP, von Minckwitz G, Ponta H, Herrlich P. CD44 variant exon epitopes in primary breast cancer and length of survival. Lancet. 1995; 345:615–619. PMID: 7534855.

30. Ristamaki R, Joensuu H, Soderstrom KO, Jalkanen S. CD44v6 expression in non-Hodgkin's lymphoma: an association with low histologic grade and poor prognosis. J Pathol. 1995; 176:259–267. PMID: 7545748.
31. Kainz C, Kohlberger P, Sliutz G, Temfer C, Heinzl H, Reinthaller A, et al. Splice variants of CD44 in human cervical cancer stage IB to IIB. Gynecol Oncol. 1995; 57:383–387. PMID: 7539775.

32. Uhl-Steidl M, Muller-Holzner E, Zeimet AG, Adolf GR, Daxenbichler G, Marth C, et al. Prognostic value of CD44 splice variant expression in ovarian cancer. Oncology. 1995; 52:400–406. PMID: 7543667.

33. Mulder JW, Kruyt PM, Sewnath M, Oosting J, Seldenrijk CA, Weidema WF, et al. Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet. 1994; 344:1470–1472. PMID: 7526103.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download