Abstract
We report a series of epidural hematomas which cause neurologic deterioration after spinal surgery, and have taken risk factors and prognostic factors into consideration. We retrospectively reviewed the database of 3720 cases of spine operation in a single institute over 7 years (1998 April-2005 July). Nine patients who demonstrated neurologic deterioration after surgery and required surgical decompression were identified. Factors postulated to increase the postoperative epidural hematoma and to improve neurologic outcome were investigated. The incidence of postoperative epidural hematoma was 0.24%. Operation sites were cervical 3 cases, thoracic 2 cases, and lumbar 4 cases. Their original diagnoses were tumor 3 cases, cervical stenosis 2 cases, lumbar stenosis 3 cases and herniated lumbar disc 1case. The symptoms of epidural hematomas were neurologic deterioration and pain. After decompression, clinical outcome revealed complete recovery in 3 cases (33.3%), incomplete recovery in 5 cases (55.6%) and no change in 1 case (11.1%). Factors increasing the risk of postoperative epidural hematoma were coagulopathy from medical illness or anticoagulation therapy (4 cases, 44.4%) and highly vascularized tumor (3 cases, 33.3%). The time interval to evacuation of complete recovery group (29.3 hours) was shorter than incomplete recovery group (66.3 hours). Patients with coagulopathy and highly vascularized tumor were more vulnerable to spinal epidural hematoma. The postoperative outcome was related to the preoperative neurological deficit and the time interval to the decompression.
Postoperative spinal epidural hematomas (SEH), although rare, are classic complication of spinal surgery.1 SEH are believed to originate from the rich venous plexus of the epidural space. The most common area involved is the thoracic spine, where the epidural space is most prominent.
Postoperative epidural hematomas should be suspected in the patient who either demonstrates a new postoperative neurologic deficit or develops deficits in the immediate postoperative period that are consistent with cauda equina syndrome. But the contribution of an epidural hematoma to pain or new neurological deficit recognized immediately after initial operation is often questioned, because postoperative imaging after spinal surgery will frequently demonstrate some blood at the surgical site.1
Most surgical procedures involving the spine will develop a small, clinically insignificant epidural hematoma.2 However, SEH in this study refer to cases that develop hematomas significant enough to cause spinal cord compression and neurologic symptoms requiring 2nd operation.
These SEHs will cause spinal pain and root pain, followed by a progressive neurologic deterioration, whose features will be dependent on the level of compression.3
We analyzed a series of epidural hematomas which cause postoperative neurologic deterioration after spinal surgery or invasive procedure to investigate risk factors for the development of an epidural hematoma and prognostic factors.
We retrospectively reviewed the database of 3720 cases of spine operation in a single institute over 7 years (1998 April~2005 July). Among them, we investigated that nine patients of spinal epidural hematoma (SEH) who demonstrated neurologic deterioration or pain after initial spinal surgery or invasive procedure required surgical decompression because of an epidural hematoma. The medical records, including the past medical history, physical, preoperative and postoperative neurological examinations, symptom of the postoperative epidural hematoma as well as plain radiographs, magnetic resonance imaging (MRI) and computed tomography (CT) were reviewed.
Including established risk factors for epidural hematoma after initial operation such as multilevel surgical procedures and the presence of a preoperative coagulopathy (thrombocytopenia, coagulation factor deficiency, and anticoagulation therapy) in the literature,2 We hypothesized that intraoperative incidental durotomies, and the use of postoperative drains or drainage volume would be risk factors of postoperative SEHs. Other factors, such as intraoperative blood loss, operative time, preoperative use of anticoagulant or antiplatelet drugs, history of diabetes, and history of tobacco use in the study population, were also evaluated.
Factors postulated to improve neurologic outcome were the neurologic status before 2nd operation, the time interval between neurologic deterioration and 2nd operation.4
Most patients had been studied complete blood test including platelet counts, liver function test, coagulation function test such as prothrombin time (PT) and activated partial thromboplastin time (aPTT) preoperatively.
Neurological function was assessed using the American Spinal Injury Association (ASIA) grading system at four time points as bellows: before and immediately after initial operation, just before and after 2nd operation. Statistical analysis of factors for prognosis was performed with nonparametric statistical analysis, Mann-Whitney test using SPSS for windows software (SPSS Inc., Chicago, IL, USA, Ver 12.0).
The average age of the patients was 57.3 years (range 37 to 76), and there were 1 : 2 male to female ratio. Mean follow up period was 24.9 months postoperatively. The incidence of postoperative epidural hematoma was 0.24%. The incidence of epidural hematoma after open surgery except invasive procedure (epidural pain block) was 0.19%.
Operation sites were identified as cervical 3 cases, thoracic 2 cases, and lumbar 4 cases. Their original diagnosis treated by 1st operation were tumor 3 cases (hemangioma 1/meningioma 1/metastatic bone tumor 1), cervical stenosis 2 cases (cervical spondylosis 1/cervical ossification of posterior longitudinal ligament 1), lumbar stenosis 3 cases (degenerative spondylolisthesis 2/degenerative lumbar stenosis 1) and herniated lumbar disc 1 case (Table 1).
Seven patients underwent posterior procedures, which involved, in part, performance of a laminectomy and/or instrumentation and one patient underwent anterior procedure (Patient 2; cervical anterior fusion). Among posterior approach group, two patients (patient 3 and 8) were performed with epidural block.
The mean 1st operation time was 143.3 minutes and hemovac drain was inserted to six patients except patient 1,3 and 8.
The clinical presentation, which heralded the onset of neurologic deterioration, consisted of a sharp, severe pain at the level of the previous surgery. This was followed by radicular symptoms consisting of pain (3cases, 33.3%), bladder dysfunction (3cases, 33.3%). Soon after, motor weakness and sensory loss from spinal cord compression ensued (8cases, 88.9%). Patient 1 showed only pain on lower extremity and bladder symptom without motor and sensory dysfunction (Table 2). The patients showed feature of delayed postoperative SEH,2 defined as neurologic deterioration more than 3 days after operation, were teo patient. Patient 6 showed leg pain and weakness on the 4th day after operation and patient 9 showed paraparesis on the 5th day after operation.
The diagnosis of SEH was confirmed by MRI in seven patients and CT in two patients. The signal characteristics of the lesion included isointense or increased signal intensity on T1 -weighted image, heterogenous hyperintense on T2-weighted images. The sagittal and parasagittal images usually show a convex lens-shaped lesion (Fig. 1). After posterior procedure, the hematoma was usually located dorsally in seven patients except two patient; patient 4 and 8 showed ventrolaterally located SHE (Fig. 2). Patient 2 showed localized epidural hematoma compressing cervical cord ventrally at the level of initial surgery (Fig. 3). Patient 3 showed extensive dorsally located SEH although minimal invasive procedure (epidural block on C3, 4, 5) had been performed (Fig. 4). The clinical level of the lesion matched the radiographic level.
All patients were treated with emergent surgical evacuation of the hematoma but in a case of patient 8, we performed delayed surgery due to patient's poor general condition. The original site of the surgery was reexplored and the clot evacuated. In many cases the hematoma was liquefied and exuded from the wound under pressure. In case of patient 4, the laminectomy had to be extended to inferiorly T2 level to remove blood clot. In case of patient 2, blood clot was found intermingled with a absorbable hemostats, Surgicel (Ethicon®SARL, Rue du Puits Godet, Neuch Atel, Switzerland) resulted in expanded space occupying lesion (Fig. 3). The average time interval between neurologic deterioration and 2nd operation was 44.8 hours(ranged 5-176.7 hours, excluding patient 8). On subsequent 2nd operation, all of the cases had drains during the immediate postoperative period.
Preexisting medical history postulated to increase the postoperative SEH were found in four patients (44.4%); multiple myeloma 1 case, end stage renal disease 1 case, liver cirrhosis by hepatitis C viral infection 1case and deep vein thrombosis treated with coumadization 1 case (Table 1).
In the study of coagulation function test, two patients showed prolonged coagulation time (22.2%). Patient 3, who had been treated with coumadization for deep vein thrombosis, showed prolonged PT (INR 3.95, reference value 0.7-1.2) and upper normal value of aPTT (44.9 seconds, reference value 28-45). Patient 8 with liver cirrhosis showed prolonged PT as INR 1.36, normal aPTT as 33 seconds. The average platelet counts of this series was 235,000/µL (reference value 150,000-400,000/µL) and only one case (patient 8) showed abnormal range of platelet counts of 96,000/µL.
Surgical evacuation of SEH resulted in overall neurological improvement in our series. Short time interval to 2nd operation and incomplete neurological deterioration seemed to be related to good clinical outcome.
Clinical outcome assessed using the ASIA neurological function grades revealed complete recovery in 3 cases (33.3%; Patient 1, 2 and 3), incomplete recovery in 5 cases (55.6%; Patient 4, 5, 6, 7 and 8) and no change in 1 case (11.1%; Patient 9, Table 2).
In the complete recovery group, the average time from neurologic deterioration to the 2nd operation (symptom duration) was 29.3 hours (ranged 7-72 hours) and all of the patients were treated for degenerative disease. In the incomplete recovery group. The average symptom duration was 66.3 hours (ranged 7.5-176.7 hours) and patient 8 was excluded in this study due to delayed surgical time due to patients condition. The no change group consists of only one patient showed symptom duration as 5 hours. Although non parametric statistical analysis using Mann-Whitney test showed no difference in the symptom duration between complete and incomplete recovery group (p = 0.48), we could assume that there is a tendency of the short symptom duration affecting the good clinical outcome.
Patient 1 had experienced loss of pain after the 2nd operation. In a case of Patient 2, motor and sensory functions were normalized immediately after removal of hematoma mixed with Surgicel. Excessive use of absorbable hemostats due to massive epidural venous bleeding after decompression of ossification of posterior longitudinal ligament (OPLL) was the main cause of cord compression. Early diagnosis and evacuation of SEH for the patient 2 and 3 could lead to a complete neurologic recovery. Patient 4 had paraparesis postoperatively (ASIA B) which mildly improved as grade 4 (ASIA D) at 12 month follow-up. Patient 6 had residual weakness of left big toe dorsiflexion as grade 4, Patient 7 had residual weakness of left ankle dorsiflexion as grade 1 and improvement in sensory function at final follow up. Patient 9 (ASIA B) had no improvement in neurological function after the 2nd operation, but there was no available long term results because of death on 2.5 months after the 2nd operation (cause of death was multiple myeloma). There was some limitation to assess the improvement in clinical outcome precisely because the ASIA neurological function grades cannot represent the improvement in peripheral nerve function (cauda equina) thoroughly.
Postoperative spinal epidural hematomas, although rare, are classic complication of spinal surgery. The incidence were reported by Scavarda et al.5 and Lawton et al.6 (0.1%), Uribe et al. (0.22%).1 Uribe et al. also reported the series of delayed postoperative spinal epidural hematoma (DPOSEH) defined as neurologic deterioration more than 3 days after operation, the incidence as 0.17%.
In our series, SEH occurred at a rate of 0.24% and 0.19% excluding SEH after invasive procedure. 2 cases (0.05%) showed a feature of DPOSEH. We also examined 5 patients (excluded in our study) with hematoma developed in the soft tissues after cervical anterior fusion. They showed swelling on the neck and respiratory distress as initial symptom of hematoma in a common feature. After surgically treated, they were recovered normally and showed no neurological impairment.
The decision to reoperate after spinal surgery because of neurologic deterioration with a support of complementary radiologic investigation is common in the present time. Epidural hematoma should be suspected in patients presenting with a new postoperative deficit,2 and rapid surgery is a determinant factor of a full neurologic recovery.5 However, postoperative cord dysfunction may also be caused by spinal cord injury during surgery and incorrect alignment of the spine associated with graft complication.7 So the accurate radiologic diagnosis before reoperation is prerequisite for successful treatment. MRI has replaced computed tomography or myelography as the screening test for the diagnosis of SEH. The sagittal MRI and parasagittal images usually demonstrate that the lesion is present in the dorsal epidural space and in some cases extends laterally. The MRI features were quite specific for hemorrhage, including isointense signal on T1-weighted images, high signal on T2-weighted images in acute cases and increased signal intensity on both T1 - and T2 -weighted images in subacute cases.8-11 In a case of hyperacute stage of the hematoma, contrast-enhanced MR images may be useful. After IV contrast(Gadolinium) material administration, sizeable dotted enhancement was noted in the hematoma, thus suggesting the extravasation of contrast-enhanced blood. Furthermore, a sizeable enhancement in the hyperacute stage of the hematoma itself might indicate continuing bleeding.12 MRI was more helpful than CT in defining the extent, volume and precise location of epidural hematoma in our series.
Multilevel surgical procedures and the presence of a preoperative coagulopathy are established significant risk factors for epidural hematoma after spinal surgery.2 Groen et al.8 reported larger exposures of the epidural space may increase the risk of insidious bleeding from the prominent internal vertebral venous plexus and subsequently form a hematoma as well. Spontaneous epidural hematomas have been reported in those with liver disease and coagulopathy.13 In our series, only 2 patients had abnormal coagulation function tests at the time of the initial operation. Patient 3 had been treated with coumadization for deep vein thrombosis and patient 8 had liver cirrhosis with HCV infection. In the normal coagulation function test group, patient 5 had a medical history of end stage renal disease and treated with hemodialysis, patient 9 was diagnosed as metastatic vertebral tumor from multiple myeloma assumed as having inadequate coagulation function, but not revealed at preoperative coagulation function test. From a results of our study, we assume that the primary disease having a tendency of bleeding, such as tumor with high vascularity (3 cases in our study), may contribute to increase the risk of spinal epidural hematoma. Although blood loss during operation, 1278 mL in average, was larger than usual spinal operation, there was insufficient evidence of assuming it as a risk factor of spinal epidural hematoma. Although the neurosurgeon was confident at the end of the initial surgery, inadequate hemostasis during this procedure cannot be definitely ruled out as a causal factor. Extra precautions for meticulous hemostasis during the surgical procedure should be considered in patients who require multilevel decompressions and/or have a preoperative coagulopathy.
The postoperative outcome after decompression was thought to be related to the preoperative neurological deficit (complete or incomplete motor or sensory deficit) and time interval to the decompression. Delamarter et al.14 demonstrated in a dog study that when compression of the spinal cord lasted 6 hours there was no neurologic recovery and that there was progressive necrosis of the spinal cord. Vandermeulen et al.15 found that most patients with an SEH that were decompressed surgically within 8 hours made good or partial recovery of neurologic function. In our study, the average operative interval of complete recovery group (29.3 hours) was shorter than incomplete recovery group (66.3 hours) but it was too small number of population to prove the risk factor. Immediate surgical evacuation of the hematoma resulted in neurological improvement in eight of our nine patients, demonstrating that the preoperative ASIA neurological grade may be helpful as a predictor of neurologic outcome of all postoperative symptomatic epidural hematoma patients. 1 case (patient 9) with preoperative complete neurologic deficit had no improvement at the last follow up. Our findings are consistent with other clinical reports describing the relationship of rapidity of surgical decompression, neurologic grade and outcome.4,16-20
By the results of our study, it is important to diagnose an epidural hematoma as soon as possible and to evacuate hematoma immediately. The retrospective design and the rarity of the complication limit this study. The rare occurrence of this complication precludes any other reasonable study design unless there is a multicenter effort. The incidence of coagulopathies may not be accurate because some coagulopathies may be undiagnosed or unreported. Future studies to elucidate more risk factors and factors predisposed to improve surgical outcome would benefit from a multicenter effort to address the rare occurrence of postsurgical epidural hematomas.
Figures and Tables
 | Fig. 1Dorsally located epidural hematoma in thoracic spine (Patient 9).The signal characteristics of the epidural hematoma lesion included isointense or increased signal intensity on T1-weighted image, heterogenous intensity on T2-weighted images. The sagittal and parasagittal images usually show a convex lens-shaped lesion. |
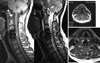 | Fig. 3Patient 2 showed localized epidural hematoma mixed with absorbable hemostats, Surgicel compressing cervical cord ventrally at the level of initial surgery. |
 | Fig. 4Slightly increased signal on T1-weighted images, high signal on T2-weighted images in acute epidural hematoma case. Patient 3 showed extensive dorsally located SEH although minimal invasive procedure (epidural block on C3, 4, 5) had been performed. |
References
1. Uribe J, Moza K, Jimenez O, Green B, Levi AD. Delayed postoperative spinal epidural hematomas. Spine J. 2003. 3:125–129.
2. Kou J, Fischgrund J, Biddinger A, Herkowitz H. Risk factors for spinal epidural hematoma after spinal surgery. Spine. 2002. 27:1670–1673.
3. Johnston RA. The management of acute spinal cord compression. J Neurol Neurosurg Psychiatry. 1993. 56:1046–1054.
4. Foo D, Rossier AB. Preoperative neurological status in predicting surgical outcome of spinal epidural hematomas. Surg Neurol. 1981. 15:389–401.
5. Scavarda D, Peruzzi P, Bazin A, Scherpereel B, Gomis P, Graftieaux JP, et al. Postoperative spinal extradural hematomas. 14 cases. Neurochirurgie. 1997. 43:220–227.
6. Lawton MT, Porter RW, Heiserman JE, Jacobowitz R, Sonntag VK, Dickman CA. Surgical management of spinal epidural hematoma: relationship between surgical timing and neurological outcome. J Neurosurg. 1995. 83:1–7.
7. Yonenobu K, Hosono N, Iwasaki M, Asano M, Ono K. Neurologic complications of surgery for cervical compression myelopathy. Spine. 1991. 16:1277–1282.
8. Groen RJ, Ponssen H. The spontaneous spinal epidural hematoma. A study of the etiology. J Neurol Sci. 1990. 98:121–138.
9. Boukobza M, Guichard JP, Boissonet M, George B, Reizine D, Gelbert F, et al. Spinal epidural haematoma: report of 11 cases and review of the literature. Neuroradiology. 1994. 36:456–459.
10. Bernsen PL, Haan J, Vielvoye GJ, Peerlinck KM. Spinal epidural hematoma visualized by magnetic resonance imaging. Neuroradiology. 1988. 30:280.
11. Rothfus WE, Chedid MK, Deeb ZL, Abla AA, Maroon JC, Sherman RL. MR imaging in the diagnosis of spontaneous spinal epidural hematomas. J Comput Assist Tomogr. 1987. 11:851–854.
12. Nawashiro H, Higo R. Contrast enhancement of a hyperacute spontaneous spinal epidural hematoma. AJNR Am J Neuroradiol. 2001. 22:1445.
13. Laglia AG, Eisenberg RL, Weinstein PR, Mani RL. Spinal epidural hematoma after lumbar puncture in liver disease. Ann Intern Med. 1978. 88:515–516.
14. Delamarter RB, Sherman J, Carr JB. Pathophysiology of spinal cord injury. Recovery after immediate and delayed decompression. J Bone Joint Surg Am. 1995. 77:1042–1049.
15. Vandermeulen EP, Van Aken H, Vermylen J. Anticoagulants and spinal-epidural anesthesia. Anesth Analg. 1994. 79:1165–1177.
16. Beatty RM, Winston KR. Spontaneous cervical epidural hematoma. A consideration of etiology. J Neurosurg. 1984. 61:143–148.
17. Cooper DW. Spontaneous spinal epidural hematoma. Case report. J Neurosurg. 1967. 26:343–345.
18. Dickman CA, Shedd SA, Spetzler RF, Shetter AG, Sonntag VK. Spinal epidural hematoma associated with epidural anesthesia: complications of systemic heparinization in patients receiving peripheral vascular thrombolytic therapy. Anesthesiology. 1990. 72:947–950.
19. Payne DH, Fischgrund JS, Herkowitz HN, Barry RL, Kurz LT, Montgomery DM. Efficacy of closed wound suction drainage after single-level lumbar laminectomy. J Spinal Disord. 1996. 9:401–403.
20. Dolan EJ, Tator CH, Endrenyi L. The value of decompression for acute experimental spinal cord compression injury. J Neurosurg. 1980. 53:749–755.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download