Abstract
In neuromuscular disease (NMD) patients with progressive muscle weakness, respiratory muscles are also affected and hypercapnia can increase gradually as the disease progresses. The fundamental respiratory problems NMD patients experience are decreased alveolar ventilation and coughing ability. For these reasons, it is necessary to precisely evaluate pulmonary function to provide the proper inspiratory and expiratory muscle aids in order to maintain adequate respiratory function. As inspiratory muscle weakening progresses, NMD patients experience hypoventilation. At this point, respiratory support by mechanical ventilator should be initiated to relieve respiratory distress symptoms. Patients with adequate bulbar muscle strength and cognitive function who use a non-invasive ventilation aid, via a mouthpiece or a nasal mask, may have their hypercapnia and associated symptoms resolved. For a proper cough assist, it is necessary to provide additional insufflation to patients with inspiratory muscle weakness before using abdominal thrust. Another effective method for managing airway secretions is a device that performs mechanical insufflation-exsufflation. In conclusion, application of non-invasive respiratory aids, taking into consideration characterization of respiratory pathophysiology, have made it possible to maintain a better quality of life in addition to prolonging the life span of patients with NMD.
Respiratory complications are a leading cause of morbidity and mortality in neuromuscular diseases (NMD). Patients with NMD are known to develop restrictive pulmonary disease pattern due to the progressive weakening of respiratory muscles.1-3 Periodic monitoring of lung volumes and maximal respiratory pressures to assess the strength of the respiratory muscles is necessary for these patients. Muscle weakness results in respiratory complications and bring about alveolar hypoventilation, eventually inducing hypercapnia.3-5 When hypercapnia occurs, patients experience symptoms such as morning headache, nightmares, daytime somnolence, and a decreased attention span. The fundamental respiratory problems NMD patients have are decreased alveolar ventilation and coughing ability. For these reasons, it is necessary to perform a precise evaluation of pulmonary function in order to provide the proper inspiratory and expiratory muscle aids to maintain adequate respiratory function.
In NMD patients with progressive muscle weakness, respiratory muscles are also affected and hypercapnia can increase gradually along with the disease. Even in the non-progressive form of the disease, muscle weakness can be aggravated by the overall aging process and hypercapnia is associated with aging as well.6 If any kind of respiratory complications occur in these patients, the respiratory work load increases, which can induce decompensation of respiratory muscles. In such cases, supportive ventilation should be provided to avoid respiratory muscle fatigue.7 If support is not provided, acute respiratory failure can be induced due to the abrupt fall in pH. Management of respiratory muscle fatigue is the key factor in the respiratory care of NMD. Weaning from ventilatory support after resolution of respiratory complications can be frustrating for a patient with advanced respiratory muscle weakness because of recurring respiratory muscle fatigue. In this situation, a patient may need reintubation and supportive ventilation repeatedly and may eventually receive a tracheostomy tube for long term support. In such patients, instead of totally weaning them, it would be better to provide noninvasive ventilatory support via nasal mask or mouthpiece after removal of the intubation tube. By providing ventilatory aid through noninvasive means, we can prevent respiratory muscle decompensation.7
When airway secretions increase due to respiratory tract infection, normal people can clear out the secretions through coughing and they seldom develop further complications such as pneumonia.8 Patients with NMD, however, tend to develop atelectasis and pneumonia easily due to an ineffective cough.9 The further expiratory muscle weakness has progressed, the greater the likelihood of reduced coughing ability to clear out secretions is. In addition to this, reduced respiratory compliance leading to contracture of the lung parenchyme and chest wall affects the inspiratory phase of coughing.10,11 Patients with limited lung expansion due to the contracture of the lung parenchyme find it more difficult to clear respiratory secretions.12 This can cause further problems when coughing assistance is needed. In order to induce effective coughing, sufficient inhalation of air should be first established. A precise assessment of the patient and a thorough understanding of the respiratory pathophysiology associated with NMD can be the basis of ventilation assistance needed to enable effective clearance of airway secretions.
Respiratory failure in NMD patients is not attributed to problems in the lung parenchyme, but to the weakened respiratory muscles. Adequate pulmonary evaluation considering this should be taken. Along with the basic pulmonary function evaluation, additional assessment and precautions for patients are as follows.
In many NMDs, weakness involves not only the skeletal muscles in extremities but also the respiratory muscles. Respiratory muscle weakness eventually results in overall respiratory dysfunction, interrupting the activities of daily life. In many cases, respiratory distress symptoms do not manifest themselves along with the progression of respiratory muscle weakness.13 For this reason, periodic evaluation of respiratory muscle function is needed in the management of NMDs. Maximal static pressure, the measuring of maximal inspiratory and expiratory pressure, is an indirect way to assess respiratory muscle power. It shows subtle changes associated with early stages of disease when the standard spirometer measurement does not detect any abnormalities.2,4 Inspiratory muscle weakness diminishes the capacity of lungs and the chest wall to expand.14 However, total lung capacity does not begin decreasing until the inspiratory muscle power declines to less than 50% of normal.13 Consequently, except in cases with advanced muscle weakness, measuring maximum inspiratory pressure is recommended for early detection of inspiratory muscle weakness.14 Maximum expiratory pressure is measured by total lung capacity, where the patient takes the deepest breath possible then blows out all of the air through a cylindrical mouthpiece into a static pressure meter. Similarly, maximum inspiratory pressure is measured by residual volume, where the patient blows out all of their air then inhales as much air as possible. For NMD patients experiencing respiratory muscle weakness, measuring maximum static pressure is a more suitable landmark for assessing patients and establishing further therapeutic plans.3,15
Measuring VC is the most fundamental landmark in pulmonary function evaluation that helps to make further therapeutic plans. The VC values can show variations according to a patient's position or indicate deterioration in their respiratory muscle function. Patients with amyotrophy lateral sclerosis (ALS) who have diaphragm weakness show greater value in sitting position.16,17 In contrast, cervical cord injury patients lacking diaphragm weakness have a higher VC measurement when in a supine position.18-20 Thus, NMD patients should have their VC measured both in sitting and supine positions because of the possible measurement variations. Lower cervical cord injury patients mainly breathe through the diaphragm due to intercostal or abdominal muscle paralysis.18,19 Paralysis of the expiratory muscle group will not trigger contraction of expiratory muscles when trying to breathe out air after full inspiration. Therefore, expiration while in a sitting position occurs passively by deflation of the fully inhaled lungs and thoracic wall through recoiling. In addition, descended abdominal contents, along with gravity, reduce the excursion of the diaphragm.18-20 This could explain the reduced VC measurement seen in a sitting position versus supine. In many ALS patients, diaphragm weakness is frequently accompanied in accordance with the progressive respiratory muscle weakness. In a supine position, increased pulmonary circulation triggers a reduction in the air volume inhaled into the thorax. This, in conjunction with compression of the diaphragm by abdominal contents, induces a decrease in VC. This phenomenon is also observed in normal healthy people. Previous studies report that normal healthy people experience a decrease in VC by 7.5% when in a supine position.17,21 In NMDs with progressive muscle weakness like ALS, VC in a supine position is closely related to the progressive weakness of the diaphragm. Discrepancies in VC measurements due to the different positions can be a possible landmark that reflects the degree of diaphragmatic weakness.
In addition to positional variations, the use of spinal orthosis, which is used to correct scoliosis in NMD patients, can limit thoracic movements. VC should be measured with the braces on and off to determine the application of the orthosis.22
Weakened respiratory muscles can neither expand the lung up to its maximal capacity nor collapse the lung to its minimal residual volume.23 If the thoracic wall does not experience a full expansion for a long period of time, the thoracic muscle component shortens and muscle fibrosis progresses, all of which reduce the compliance of thoracic wall. Compliance of the lung parenchyme may be reduced by microatelectasis of alveoli.23,24 Changes in compliance may cause serious problems in maintaining pulmonary health by reducing the capacity to cough and clear secretions.10 Therefore, measuring the maximum insufflation capacity and comparing it to actual VC can be an indirect method to determine the compliance of the respiratory system.25
In order to measure MIC, a patient is asked to inhale as much air as possible, then an extra volume of air is delivered by a manual resuscitator bag using either an oronasal mask or a mouthpiece. After holding in the additional air, the patient is then asked to blow out as much air as possible through the spirometer.22,25
Coughing is one of the most important protective mechanisms in our body. It clears out airway secretions to prevent further complications such as pneumonia.8 Reduced coughing capacity, along with respiratory muscle weakness, may cause respiratory complications by not allowing satisfactory removal of bronchial secretions.9 Coughing capacity can be assessed by having the person cough as forcefully as possible through the peak flow meter. Only people who show PCF values greater than 160 L/min, whether unassisted or manually assisted, can efficiently eliminate bronchial secretions. People with maximum assisted PCF values of 200-250 L/min, whether unassisted or manually assisted, under normal conditions usually have difficulty maintaining the minimum assisted PCF value required for removal of secretion under conditions of respiratory infection or general anesthesia.9,26 Therefore, measuring PCF values and establishing further treatment plans following PCF measurement is fundamental for NMD patients.
NMD patients with weakened respiratory muscle power are often exposed to a state of chronic hypoventilation. The acute onset of hypercapnia decreases systemic pH, triggering the respiratory drive by inducing deep breathing. However, in chronic hypercapnia, the triggering effect is somewhat muted due to the compensatory work of the kidney.27 Providing only oxygen to raise the SaO2 to a patient in this condition may induce more shallow breath, thereby aggravating hypercapnia and eventually causing CO2 narcosis.27 Accordingly, treatment of NMD patients with oxyhemoglobin desaturation should be initiated with consideration of their CO2 status.
Arterial blood gas analysis (ABGA) is generally performed to assess the overall ventilatory state of the patient. However in neuromuscular disease, serial measurements of SaO2 and EtCO2, under different circumstances with a pulse oxymeter and capnometer, is recommended for a more complete understanding of the ventilatory state.22 For instance, an ALS patient with weak diaphragm movement shows greater VC in a sitting position than a supine position.16,17 Conversely, a cervical cord injury patient has a greater VC value in a supine position.18,20 Even in the same patient, ventilatory drive is depressed when sleeping.28 Measurements should also occur when the patient is in different states of wakefulness, due to the depression of the ventilatory drive during sleep. The differences in values in this set of measurements are as important as understanding measurement variation due to differences in position. Arterial blood gas analysis is an invasive method and the associated pain may induce hyperventilation, thereby altering the data. Therefore, a non-invasive method of measurement can provide superior information for management plans.
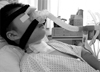
As inspiratory muscle weakness progresses, NMD patients experience hypoventilation symptoms such as sleeping disturbance, morning headache, daytime somnolence, nightmare, and anxiety due to hypercapnia. At this point, ventilation assist by mechanical ventilator should be initiated to relieve respiratory distress. Conventional invasive methods such as intubation or an indwelling tracheostomy tube can be a psychological burden for both the patients and their caregivers.29 Even when mechanical ventilator is needed, physicians and guardians, as well as patients, tend to hesitate and postpone initiating treatment due to the invasiveness and subsequent psychological despair. This causes the ventilatory status of the patient to deteriorate, thereby necessitating the need to undergo an invasive method for management of their serious condition. If a non-invasive ventilatory assist method, through a mouthpiece or a nasal mask, is provided to the patient at the beginning as an alternative choice to relieve the respiratory distress symptoms, patient would accept the use of mechanical ventilator more easily. In most cases, non-invasive ventilatory assist is sufficient to resolve hypercapnia and its associated symptoms.30,31 Intermittent positive pressure ventilation (IPPV) is a commonly used non-invasive assist. These types of assist can be successfully applied to a patient with very weak respiratory muscles if they have adequate bulbar muscle power. The quality of life for a tracheostomized ventilator user can be much improved by switching to non-invasive IPPV.32,33 Even when non-invasive IPPV can no longer substitute for invasive IPPV in some patients, it at least is a way to postpone tracheostomy without hypercapnia symptoms up to that point in time (Fig. 1).
Studies comparing the results of noninvasive versus invasive ventilatory assist have reported patients that received non-invasive IPPV showed fewer respiratory complications, lower admission rates, and shorter duration in their hospital stay when admitted.29,30 Another study comparing these two methods has reported that people generally favor the non-invasive method and this method is superior in aspects of convenience, safety, general comfort, speech, preservation of swallowing function, sleeping, and appearance.29,31 Research on the pathophysiology of applying noninvasive ventilator has determined the use of non-invasive ventilator can increase the maximum inspiratory pressure in patients with chronic alveolar hypoventilation as well as temporally increase VC.34,35 In addition, the study also reported several other possible advantages such as relieving alveolar hypoventilation symptoms, normalizing the value of ABGA, increasing endurance of respiratory muscles, reducing the admission rate and the occurrence of concomitant respiratory complications, and delaying the time of tracheostomy, all by applying non-invasive ventilation.30,31,36,37 Despite these advantages, non-invasive ventilator cannot be applied to all patients with ventilatory failure. Typical examples are patients with cognitive impairment and those with severe bulbar muscle palsy.32 Patients with impaired cognitive function can not cooperate well enough to perform assistive coughing or use assistive devices to remove airway secretions. This inability to comply can put the patients in dangerous condition.32 In order to apply non-invasive ventilation without undergoing tracheostomy, the bulbar muscle should be spared in some measure.32 Patients with bulbar muscle palsy, such as bulbar dominant ALS patients, find it difficult to increase intrapleural pressure with the glottis closed during an assistive coughing maneuver.38 In this case, it is not possible to remove airway secretions without using a device or patients would experience recurrent saliva aspiration, often a major cause of aspiration pneumonia.33 It is inevitable for such a patient to undergo tracheostomy and have invasive ventilatory assistance applied.33
Mechanical ventilators can be classified as a body ventilator which directly put pressure on body for mechanical ventilation and a positive pressure ventilator which directly delivers air into trachea. Body ventilators had once been the first line therapy as a long term assistive ventilation device. But with the introduction of positive pressure ventilators in the mid 1950s, body ventilators became less attractive because of their limited portability and patient care.39 Portable ventilators used in non-invasive IPPV can be divided into two categories: pressure limited and volume limited ventilators. Volume limited ventilators deliver a preset volume of air regardless of air leakage through the mouth or nose and the maximal airway pressure reading on the gauge of the ventilator depends on the volume of delivered air, leakage at the interface, and elasticity of the lung. PLV series (Respronics, USA), LP-10 (Aequitron medical Inc., USA), and LTV series (Pulmonetic Systems, Inc., USA) are examples of commonly used volume limited portable ventilator. The most common pressure limited ventilator is bi-level positive airway pressure (BiPAP) device. It is possible to preset the inspiratory and expiratory positive pressure separately on a BiPAP and the difference between these two pressures is considered the degree of assistive ventilation. In general, the difference between pressures is set between 5-7 cmH2O. Although this setting is suitable for people with a obstructive pattern respiratory disease such as sleep apnea, it cannot help those who are in a chronic alveolar hypoventilative state due to weakened respiratory muscles.33 Pressure limited ventilators can neither deliver sufficient air to those with atelectasis or increased airway resistance due to endotracheal secretions, nor provide the capability to perform air stacking to maintain pulmonary compliance. Therefore, it is recommended for NMD patients to use volume limited ventilators for non-invasive mechanical ventilation.33
GPB is a breathing maneuver that requires gulping boluses of air into the lung by using the tongue and pharyngeal muscles. It can be useful for the patient with ventilatory insufficiency and is especially helpful when mechanical ventilator stops working unexpectedly.33 GPB consists of 6-9 gulps of air, with 60-200 mL of air in each gulp.
Expiratory muscle aids are either a maneuver or a mechanical device used to remove endotracheal secretions. Proper coughing is prerequisite to clearing endotracheal secretions or to removing a mucus plug. The PCF of normal, healthy people reads above 6-12 L/sec. Deep inspiration and sufficient intrapleural pressure are required to induce the normal coughing process.40 Therefore, insufflating additional pre-cough volume is essential for the patient with decreased VC to induce effective coughing.11,41 A PCF over 160 L/min should be maintained to eliminate endotracheal secretions effectively.42,43 There are diverse methods and devices for managing airway secretions, however, this session will primarily focus on the methods especially useful in NMDs.
Coughing is an essential function for eliminating tracheal secretions, and reduced function may cause respiratory complications such as pneumonia. Eventually, defective coughing may be a primary cause of mortality in NMD patients with inspiratory and expiratory muscle weakness or bulbar muscle palsy. Therefore, a proper coughing assist should be provided to those NMD patients with respiratory muscle weakness. A manually assisted cough is the most commonly used assisted coughing method. After taking a deep breath, the patient is asked to cough as forcefully as possible while a simultaneously timed abdominal thrust is performed. Although taking in a sufficient volume of air should come before coughing,11,41 many NMD patients find it difficult to inhale enough air due to the weakened inspiratory muscles. Therefore, it is necessary to provide additional insufflation through a manual resuscitator bag to those with inspiratory muscle weakness before trying the abdominal thrust.11,41
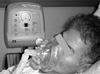
Cough assist™ (JH Emerson Co., Cambridge, MA) operates under the same principle as that of a vacuum cleaner, which generates strong expiratory flow through an instant application of negative pressure after maximum insufflation of the lung with positive pressure. Patients can clear airway secretions easily through applying Cough assist™ via an oronasal mask without undergoing tracheostomy.41 It can be a more efficient way of clearing secretions for those with a tracheostomy tube than using manual suction by a catheter. Cough assist™ eliminates respiratory secretions by alternating 40 cmH2O of positive pressure with -40 cmH2O of negative pressure, which generates approximately 10 L/sec of air flow velocity because of the pressure difference.11,44 It is an essential device for non-invasive mechanical ventilator users who have very weak coughing capacity because clearing airway secretions is possible only by applying Cough assist™ via oronasal mask.41,45 The effect of this kind of device has already been proven through many experimental studies. In an experiment, radioactive material was inhaled into the lung of a dog and, after a six-minute application of the cough assisting device, the inhaled material had been completely removed.46 In another study, values of PCF in several different situations were compared and the authors reported the measured PCF values were much higher in the group using the cough assisting device than the measurements taken in the groups using different manual assisted coughing techniques.30,41,47 Cough assist™ not only generates a higher PCF, thereby increasing the overall removal of bronchial secretions, but also eliminates the irritation and/or damage to the airway caused by catheter suction. Furthermore, when applied postoperatively, it can clear bronchial secretions without inducing wound site pain caused by generating an expiratory muscle contraction (Fig. 2).
As with other rehabilitative management program applied in different fields of medicine, active application of a pulmonary rehabilitation program can actually relieve patients' respiratory symptoms, prevent possible complications, and eventually improve the quality of life for the patient. Until now, NMD patients with ALS, myopathy, or spinal muscular atrophy, those in greater need of pulmonary rehabilitation, are often overlooked due to preoccupation with the nature of their disease. People tend to think these sets of patients are untreatable. Their disease is incurable, but their symptoms are treatable. We can assist them in many ways, especially in the management of their pulmonary complications. If there is an accurate understanding of the disease's pathophysiology and a thorough assessment of the patient occurs early on, minimization of likely respiratory complications can be possible through proper ventilatory and coughing support. Reduced respiratory complications can actually decrease the mortality rate of NMD patients. Together with the medical advantages, a patient's quality of life can be greatly enhanced by adopting non-invasive aids even if the aid will be used long term. In conclusion, use of non-invasive respiratory aids, taking into account the characterization of respiratory pathophysiology, have made it possible to enhance quality of life as well as prolong the life span of NMD patients.
Figures and Tables
References
1. Gibson GJ, Pride NB, Davis JN, Loh LC. Pulmonary mechanics in patients with respiratory muscle weakness. Am Rev Respir Dis. 1977. 115:389–395.
2. Griggs RC, Donohoe KM, Utell MJ, Goldblatt D, Moxley RT 3rd. Evaluation of pulmonary function in neuromuscular disease. Arch Neurol. 1981. 38:9–12.
3. Smith PE, Calverley PM, Edwards RH, Evans GA, Cambell EJ. Practical problems in the respiratory care of patients with muscular dystrophy. N Engl J Med. 1987. 316:1197–1205.
4. Lynn DJ, Woda RP, Mendell JR. Respiratory dysfunction in muscular dystrophy and other myopathy. Clin Chest Med. 1994. 15:661–674.
5. Schramm CM. Current concepts of respiratory complications of neuromuscular disease in children. Curr Opin Pediatr. 2000. 12:203–207.
6. Braun NM, Arora NS, Rochester DF. Respiratory muscle and pulmonary function in polymyositis and other proximal myopathies. Thorax. 1983. 38:616–623.
7. Bach JR, Alba AS. Management of chronic alveolar hypoventilation by nasal ventilation. Chest. 1990. 97:52–57.
8. Leith DE. Brain JD, Proctor D, Reid L, editors. Cough. Lung biology in health and disease. 1977. New York: Marcel Dekker;545–592.
9. Bach JR, Ishikawa Y, Kim H. Prevention of pulmonary morbidity for patients with Duchenne muscular dystrophy. Chest. 1997. 112:1024–1028.
10. Bach JR, Kang SW. Disorders of ventilation weakness, stiffness, and mobilization. Chest. 2000. 117:301–303.
11. Kang SW, Kang YS, Moon JH, Yoo TW. Assisted Cough and Pulmonary Compliance in Patients with Duchenne Muscular Dystrophy. Yonsei Med J. 2005. 46:233–238.
12. Kang SW, Bach JR. Maximum insufflation capacity: vital capacity and cough flows in neuromuscular disease. Am J Phys Med Rehabil. 2000. 79:222–227.
13. Kreitzer SM, Saunders NA, Tyler HR, Ingram RH Jr. Respiratory muscle function in amyotrophic lateral sclerosis. Am Rev Respir Dis. 1978. 117:437–447.
14. McCool FD, Tzelepis GE. Inspiratory muscle training in the patient with neuromuscular disease. Phys Ther. 1995. 75:1006–1014.
15. McDonald CM, Abresch RT, Carter GT, Flwler WM, Johnson ER, Kilmer DD, Sigford BJ. Profiles of neuromuscular diseases: Duchenne muscular dystrophy. Am J Phys Med Rehabil. 1995. 74:S70–S92.
16. Lechtzin N, Wiener CM, Shade DM, Clawson L, Diette GB. Spirometry in the supine position improves the detection of diaphragmatic weakness in patients with amyotrophic lateral sclerosis. Chest. 2002. 121:436–422.
17. Varrato J, Siderowf A, Damiano P, Gregory S, Feinberg D, McCluskey L. Postural change of forced vital capacity predicts some respiratory symptoms in ALS. Neurology. 2001. 57:357–359.
18. Baydur A, Adkins RH, Milic-Emili J. Lung mechanics in individuals with spinal cord injury: effects of injury level and posture. J Appl Physiol. 2001. 90:405–411.
19. Estenne M, De Troyer A. The effects of tetraplegia on chest wall statics. Am Rev Respir Dis. 1986. 134:121–124.
20. Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil. 2003. 82:803–814.
21. Allen SM, Hunt B, Green M. Fall in vital capacity with posture. Br J Dis Chest. 1985. 79:267–271.
22. Bach JR. Bach JR, editor. Respiratory considerations. Guide to the evaluation and management of neuromuscular disease. 1999. Philadelphia: Hanley & Belfus;67–87.
23. Estenne M, Heilporn A, Delhez L, Yerault JC, De Troyer A. Chest wall stiffness in patients with chronic respiratory muscle weakness. Am Rev Respir Dis. 1983. 128:1002–1007.
24. Estenne M, Gevenois PA, Kinnear W, Soudon P, Heilporn A, De Troyer A. Lung volume restriction in patients with chronic respiratory muscle weakness: the role of microatelectasis. Thorax. 1993. 48:698–701.
25. Kang SW, Bach JR. Maximum insufflation capacity. Chest. 2000. 118:61–65.
26. Hanayama K, Ishikawa Y, Bach JR. Amyotrophic lateral sclerosis: successful treatment of mucus plugging by mechanical insufflation-exsufflation. Am J Phys Med Rehabil. 1997. 76:338–339.
27. Beachey W. Wilkins RL, Stroller JK, Scanlan CL, editors. Regulation of breathing. Egan's fundamentals of respiratory care. 2003. 8th ed. St. Louis: Mosby;297–305.
28. Bach JR. Bacg JR, editor. Physiology and pathophysiology of hypoventilation: ventilatory vs oxygenation impairment. Noninvasive mechanical ventilation. 2002. Philadelphia: Hanley & Belfus;25–43.
29. Bach JR. A comparison of long-term ventilatory support alternatives from the perspective of the patient and caregiver. Chest. 1993. 104:1702–1706.
30. Cazzolli PA, Oppenheimer EA. Home mechanical ventilation for amyotrophic lateral sclerosis: nasal compared to tracheostomy-intermittent positive pressure ventilation. J Neurol Sci. 1996. 139:123–128.
31. Gomez Merino E, Bach JR. Duchenne muscular dystrophy: prolongation of life by noninvasive ventilation and mechanically assisted coughing. Am J Phys Med Rehabil. 2002. 81:411–415.
32. Bach JR. Indications for tracheostomy and decannulation of tracheostomized ventilator users. Monaldi Arch Chest Dis. 1995. 50:223–227.
33. Bach JR. Bach JR, editor. Noninvasive ventilation. Guide to the evaluation and management of neuromuscular disease. 1999. Philadelphia: Hanley & Belfus;89–122.
34. Ellis ER, Bye PT, Bruderer JW, Sullivan CE. Treatment of respiratory failure during sleep in patients with neuromuscular disease, positive-pressure ventilation through a nose mask. Am Rev Respir Dis. 1987. 135:148–152.
35. Vianello A, Bevilacqua M, Salvador V, Cardaioli C, Vincenti E. Long-term nasal intermittent positive pressure ventilation in advanced Duchenne's muscular dystrophy. Chest. 1994. 105:445–448.
36. Bach JR. Amyotrophic lateral sclerosis: prolongation of life by noninvasive respiratory AIDS. Chest. 2002. 122:92–98.
37. Goldstein RS, DeRosie JA, Avendano MA, Dolmage TE. Influence of noninvasive positive pressure ventilation on inspiratory muscles. Chest. 1991. 99:408–415.
38. Bach JR, Saporito LR. Criteria for extubation and tracheostomy tube removal for patients with ventilatory failure: a different approach to weaning. Chest. 1996. 110:1566–1571.
39. Bach JR. Bacg JR, editor. The history of mechanical ventilation and respiratory muscle aids. Noninvasive mechanical ventilation. 2002. Philadelphia: Hanley & Belfus;45–72.
40. Mislinski MJ, Scanlan CL. Wilkins RL, Stroller JK, Scanlan CL, editors. Bronchial hygiene therapy. Egan's fundamentals of respiratory care. 2003. 8th ed. St. Louis: Mosby;297–305.
41. Bach JR. Mechanical insufflation-exsufflation. Comparison of peak expiratory flows with manually assisted and unassisted coughing techniques. Chest. 1993. 104:1553–1562.
42. Bach JR. Amyotrophic lateral sclerosis: predictors for prolongation of life by noninvasive respiratory aids. Arch Phys Med Rehabil. 1995. 76:828–832.
43. Bach JR, Saporito LR. Indications and criteria for decannulation and transition from invasive to non-invasive long-term ventilator support. Respir Care. 1994. 39:515–531.
44. Newth CJL, Asmler B, Anderson GP, Morley J. The effects of varying inflation and deflation pressures on the maximal expiratory deflation flow volume relationship in anesthetized Rhesus monkeys. Am Rev Respir Dis. 1991. 144:807–813.
45. Bach JR. Update and perspective on noninvasive respiratory muscle aids. Part 2: The expiratory aids. Chest. 1994. 105:1538–1544.
46. Bickerman HA. Exsufflation with negative pressure: elimination of radioopaque material and foreign bodies from bronchi of anesthetized dogs. AMA Arch Intern Med. 1954. 93:698–704.
47. Barach AL, Beck GJ. Exsufflation with negative pressure: physiologic and clinical studies in poliomyelitis, bronchial asthma, pulmonary emphysema and bronchiectasis. AMA Arch Intern Med. 1954. 93:825–841.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download