Abstract
The transition between the main subtypes of pemphigus, pemphigus vulgaris (PV), and pemphigus foliaceus (PF) has rarely been reported. Moreover, the development of PV in a patient with PF is much more unusual than that of PF in a patient with PV. We report a 48-year-old man who presented with cutaneous lesions showing the typical clinical and histological features of PF. Five years later, his skin lesions became extensive and he developed oral erosions. His condition did not respond well to steroids and azathioprine. Histological examination of a vesicle disclosed suprabasal acantholysis in contrast to the subcorneal acantholysis discovered upon initial histological evaluation. Indirect immunofluorescence revealed IgG antikeratinocyte cell surface antibodies at a titer of 1:640. The titer was 1:160 at initial diagnosis. Upon immunoblotting, the patient's serum reacted with 130 kiloDalton (kDa) and 160 kDa proteins, suggesting desmoglein (Dsg) 3 and 1, respectively. We herein report an unusual case of PV that developed from PF during the disease's flare-up.
The two major subtypes of pemphigus are pemphigus vulgaris (PV) and pemphigus foliaceus (PF). They share many similarities but they differ in pathogenesis and clinical features. Several cases in which the disease course has undergone a transition of pemphigus subtypes have been reported in the literature. A change from PF to PV, however, is more unusual than a change from PV to PF. In this report, we describe a case of PF which developed clinical and histological features consistent with PV five years after the initial diagnosis. We also attempt to review the literature on this rare transition of pemphigus subtypes.
A 48-year-old Korean man presented in October 1998 with a 5 month history of itchy skin lesions. Physical examination revealed multiple, coin- and palm-sized erythematous patches with some crusts and erosions on the scalp and trunk. Several flaccid vesicles were also seen on the trunk. However, no oral mucosal lesions were observed. Histological examination of a skin lesion on the back disclosed a subcorneal acantholytic blister (Fig. 1A). Direct immunofluorescence demonstrated intercellular IgG and C3 immune deposits. Indirect immunofluorescence showed IgG antikeratinocyte cell surface antibodies at a titer of 1:160, and this lead to the diagnosis of PF.
The patient had been treated with oral prednisolone and dapsone until he was admitted to the hospital in November 2003 because of aggravation of his skin lesions. Over this period, his disease activity had fluctuated but there was never any development of oral mucosal erosions. After his admission, azathioprine was substituted for dapsone, and his cutaneous lesions markedly improved. However, two months after discharge he was rehospitalized because of the appearance of extensive, erosive, crusted lesions and flaccid vesicles on the trunk and extremities. One week later, oral mucosal erosions were observed. Histological examination of a skin lesion on the left arm revealed suprabasal acantholysis (Fig. 1B). Indirect immunofluorescence showed IgG antikeratinocyte cell surface antibodies at a titer of 1:640.
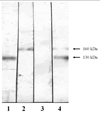
Suspecting PV transition from PF, we performed an immunoblot assay to confirm the diagnosis. With immunoblotting, the serum of the patient during the PV stage reacted with both 130 kDa and 160 kDa proteins, suggesting Dsg 3 and 1, respectively. However, the serum sample of the PF stage at initial diagnosis displayed a negative result. This is most likely due to the decreased antibody activity of the serum while it was stored during the past 5 years (Fig. 2).
In addition to the unresponsiveness of his skin lesions to steroids and azathioprine, the patient also developed a fever, indicating a nosocomial infection due to his defective skin barrier. Despite the extensive administration of intravenous antibiotics, his fever could not be controlled and his condition deteriorated. Eventually, he developed sepsis and died of acute respiratory distress syndrome 24 days after admission.
Pemphigus consists of two major distinctive subsets, PV and PF. Although both subsets display acantholytic bullae in the epidermis, PV is different from PF in that the former involves the oral mucosa and shows suprabasal acantholysis. In contrast, PF demonstrates subcorneal acantholysis and does not lead to oral lesions. On immunoblot assay, PF has anti-Dsg 1 antibodies, the mucosal-dominant type of PV has anti-Dsg 3 antibodies, and the mucocutaneous type of PV has both anti-Dsg 1 and anti-Dsg 3 antibodies.
Although the transition between PV and PF is a rare medical event, several reports have been issued on this phenomenon (Table 1). Moreover, the transition from PF to PV is less common than that of PV to PF.1-10
The mechanism of transition between PV and PF remains elusive. One possible mechanism is an epitope-spreading phenomenon which is considered as a primary autoimmune or inflammatory process that causes tissue damage by exposing immunologically hidden protein to the immune system, thereby evoking a secondary autoimmune reponse.5-7 In cases of PV transforming from PF, anti-Dsg 1 autoantibodies were initially present. Dsg 3 may be released from skin lesions and become exposed to the immune system in events such as elevation of disease activity or thymoma resection. This leads to the production of anti-Dsg 3 autoantibodies and a change in the phenotype.5,7
Immunoblotting and enzyme-linked immunosorbent assay (ELISA) have been used to demonstate the autoantibodies in pemphigus.1-10 Unlike ELISA, immunoblotting does not yield a quantitative result, and it may produce false negative results.10 When we suspected the transition in our case, we performed immunoblotting to confirm the change in autoantibodies. With immunoblotting, the serum sample during the PV stage reacted with 130 kDa and 160 kDa proteins. However, the serum sample at the time of the initial diagnosis of PF failed to react with the 160 kDa protein. This is probably due to decreased antibody activity of the serum stored at 4℃ for 5 years, and also due to the fact that the characteristics of the immunoblotting method are more qualitative than quantitative.
Although we failed to demonstrate autoantibodies in the serum samples of the initial diagnosis of PF by immunoblotting, it is evident that our patient was suffering from PF based on his clinical and histological features.
Figures and Tables
Fig. 1
Histologic features of vesicles. Subcorneal acantholysis in the PF stage (A). Suprabasal acantholysis in the PV stage (B). (Hematoxylin & Eosin, × 100).
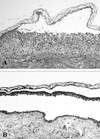
Fig. 2
The results of the immunoblot assay. The reference serum of the patient with PV (lane 1) and PF (lane 2) reacted with 130 kDa protein and 160 kDa protein, respectively. The 5 year-old serum of our patient with the PF stage showed a negative result (lane 3), whereas the serum of the PV stage reacted with 130 kDa and 160 kDa proteins (lane 4).
References
1. Hashimoto T, Konohana A, Nishikawa T. Immunoblot assay as an aid to the diagnoses of unclassified cases of pemphigus. Arch Dermatol. 1991. 127:843–847.
2. Iwatsuki K, Takigawa M, Hashimoto T, Nishikawa T, Yamada M. Can pemphigus vulgaris become pemphigus foliaceus? J Am Acad Dermatol. 1991. 25:797–800.
3. Kawana S, Hashimoto T, Nishikawa T, Nishiyama S. Changes in clinical features, histologic findings, and antigen profiles with development of pemphigus foliaceus from pemphigus vulgaris. Arch Dermatol. 1994. 130:1534–1538.
4. Chang SN, Kim SC, Lee IJ, Hong CK, Park WH. Transition from pemphigus vulgaris to pemphigus foliaceus. Br J Dermatol. 1997. 137:303–305.
5. Ishii K, Amagai M, Ohata Y, Shimizu H, Hashimoto T, Ohya K, et al. Development of pemphigus vulgaris in a patient with pemphigus foliaceus: antidesmoglein antibody profile shift confirmed by enzyme-linked immunosorbent assay. J Am Acad Dermatol. 2000. 42:859–861.
6. Komai A, Amagai M, Ishii K, Nishikawa T, Chorzelski T, Matsuo I, et al. The clinical transition between pemphigus foliaceus and pemphigus vulgaris correlates well with the changes in autoantibody profile assessed by an enzyme-linked immunosorbent assay. Br J Dermatol. 2001. 144:1177–1182.
7. Kimoto M, Ohyama M, Hata Y, Amagai M, Nishigawa T. A Case of pemphigus foliaceus which occurred after five years of remission from pemphigus vulgaris. Dermatology. 2001. 203:174–176.
8. Tsuji Y, Kawashima T, Yokota K, Tateish Y, Tomita Y, Matsumura T, et al. Clinical and serological transition from pemphigus vulgaris to pemphigus foliaceus demonstrated by desmoglein ELISA system. Arch Dermatol. 2002. 138:95–96.
9. Harman KE, Gratian MJ, Shirlaw PJ, Bhogal BS, Challacombe SJ, Black MM. The transition of pemphigus vulgaris into pemphigus foliaceus: a reflection of changing desmoglein 1 and 3 autoantibody levels in pemphigus vulgaris. Br J Dermatol. 2002. 146:684–687.
10. Toth GG, Pas HH, Jonkman MF. Transition of pemphigus vulgaris into pemphigus foliaceus confirmed by antidesmoglein ELISA profile. Int J Dermatol. 2002. 41:525–527.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download