Abstract
Although the head and neck region is recognized as the most common location for peripheral nerve sheath tumors, central involvement, particularly in the jaw bones, is quite unusual. Neurofibroma is one of the most common nerve sheath tumors occurring in the soft tissue and generally appears in neurofibromatosis 1 (NF1 or von Recklinghausen's disease). Malignant peripheral nerve sheath tumors (MPNSTs) are uncommon sarcomas that almost always arise in the soft tissue. Here, we report four cases of intraosseous peripheral nerve sheath tumors occurring in the jaw bones and compare the clinical, radiologic, and pathologic findings in order to make a differential diagnosis.
Benign tumors of the peripheral nerve sheath, particularly neurofibromas, often occur in the soft tissues of the head and neck region. However, intraosseous neurofibromas are reportedly rare, because the bones do not contain myelinated nerves or nerve sheaths within their medullary space.1 The majority of central neurofibromas occur in the mandible and 33 cases have been reported so far.2-8
Neurofibroma occurring in the jaw bones characteristically presents as a single lesion, and rarely presents as multiple lesions, such as in von Recklinghausen's neurofibromatosis (also called NF1). They often exhibit deformed and expanded nerve canals and jaw cortices as a result of the pressure of the neurofibroma's expansile growth.
Malignant tumors arising from the peripheral nerves or displaying a different degree of differentiation of the various elements of the nerve sheath, are now collectively referred to as malignant peripheral nerve sheath tumors (MPNST).9 This can replace a number of tumors that have been previously known as malignant schwannomas, neurofibrosarcomas, and neurogenic sarcomas. Although there is some controversy as to the diagnostic criteria of this tumor, a sarcoma arising from a peripheral nerve or a pre-existing neurofibroma is generally accepted to be MPNST.10 They are usually high-grade sarcomas with frequent pleomorphism, atypical mitosis, and necrosis, and should be differentiated from melanomas, spindle-cell carcinomas, and sarcomas such as malignant fibrous histiocytoma, fibrosarcoma, synovial cell sarcoma, and leiomyosarcoma.9
Peripheral nerve sheath tumors are difficult to diagnose and treat due to their rarity, their variable clinical expression pattern, and their progressive growth, even when benign. Here, we report four cases of an intraosseous peripheral nerve sheath tumor occurring in the mandible and/or maxilla with their clinical, radiographic, and histopathological characteristics.
A 10-year-old girl was evaluated by her dentist for a delayed eruption of the mandibular right first molar. During an oral examination, a bulging of the edentulous alveolar ridge was observed posterior to the partially erupted right mandibular first premolar and to the maxillary first molar (Fig. 1-A). Facial disfigurement due to the bulging of the right lower face was also noted. A physical examination revealed multiple cafe-au-lait spots in her neck, back, and axilla. The patient's mother also had cafe-au-lait spots on her right arm.
A panoramic radiograph showed two separate areas of radiolucency at the body-ramus of the right mandible and right posterior maxilla (Fig. 1-A). The teeth were impacted in the lesions. A computed tomography (CT) scan revealed two well-circumscribed radiolucent lesions and overlying cortical expansions at the mandibular bodyramus and posterior maxilla (Fig. 1-B, C). The maxillary lesion extended to the infratemporal space with destruction of the posterior maxillary wall.
After an incisional biopsy, surgical enucleations of the mandibular and maxillary lesion via intraoral approach were performed. Two specimens from the mandible (3.0 × 3.0 × 3.0 cm each) and one from the maxilla (3.5 × 4.0 × 3.5 cm) with the impacted teeth were sent for pathologic evaluation and were diagnosed as neurofibromas.
Histologically, the tumor contained interlacing bundles of elongated cells with wavy, dark-staining nuclei. The cells were intimately associated with wire-like strands of collagen. (Fig. 1-D) The stroma of the tumor was dotted with occasional mast cells, lymphocytes, and small nerve fibers throughout the tumor. Most of the tumor cells were positive for S-100 protein on immunohistochemical staining (Fig. 1-E). Although the dental follicular tissue was largely separated from the tumor, the stromal portion of the follicle had a hyalinized background, instead of myxoid nature found in normal dental follicles. However, immunostaining for S-100 protein was negative on the follicle stroma.
A 23-year-old male patient visited the oral and maxillofacial clinic for facial esthetic problems. He showed an asymmetric face with an outward bulging of the lower face. His left lower molars were absent and the overlying mucosa was expanded lingually. A physical examination revealed multiple cafe-au-lait spots at the back, arm and abdomen, which were confirmed by a dermatologist. But a familial history of neurofibromatosis was not reported.
A panoramic radiograph revealed that the mandibular left second and third molars were impacted and the inferior alveolar canal was displaced inferiorly. A relatively well-circumscribed radiolucent lesion was found around the impacted molars in the mandibular angle and ramus. A CT scan revealed a radiolucent lesion around the teeth and the surrounding buccal cortices were expanded in the mandible (data not shown).
After an incisional biopsy, the lesion was enucleated and the impacted teeth were extracted. Microscopically, the tumor consisted of spindle cells associated with collagen fibrils and a large amount of stromal mucosubstances that were similar to that observed in Case 1. Immunohistochemical staining for S-100 protein revealed a strong positive reaction on the tumor cells. The dental follicular tissue was separated from the main tumor, even though the stromal compartment was rich in hyalinized collagen. The operation wounds healed well without any complications and the patient showed no signs of recurrence during follow-ups.
A 15-year-old female patient was referred by a local dental clinic for an evaluation of radiolucency on the anterior mandible. During the clinical examinations, tooth mobility of the four lower anterior teeth and the elevation of the labial vestibule around them were noted. There were no clinical findings of neurofibromatosis. The panoramic radiograph (Fig. 2-A) and CT scan (Fig. 2-B) showed a round radiolucent lesion with an ill-defined margin and resorption of the lower anterior teeth. The lingual cortex was intact, but the labial side was perforated with a radiolucent mass. In addition, root resorption of the affected teeth was evident on the periapical dental X-ray (Fig. 2-C).
MPNST was diagnosed based on an incisional biopsy of the lesion and a wide excision of the mandible was performed. A histologic examination showed that the tumor cells were arranged in short fascicles without any nuclear palisading. The nuclei were irregular in shape and the cytoplasms were lightly stained and indistinct (Fig. 2-D and E). In addition, multinucleated giant cells and abnormal mitoses were present throughout the tumor. Immunostaining for S-100 protein was positive in the cytoplasm and the nuclei of some tumor cells, whereas HMB-45 staining was negative.
A 13-year-old girl was evaluated and referred by her dentist for an abnormal mucosal and bony defect on the right maxillary incisor region after orthodontic treatment. During the oral examination, gingival recession with tooth mobility were observed on the right maxillary incisors (Fig. 3-A). Dental radiographs showed extensive osseous defects and external root resorption of the right maxillary incisors and canine (Fig. 3-B). The CT scan revealed a poorly demarcated radiolucent lesion involving the labial, palatal and nasal sides of the region (Fig. 3-C). But the patient demonstrated no clinical sign of neurofibromatosis.
After an incisional biopsy, a transoral wide excision and anterior maxillectomy was performed. Histologically, the tumor contained spindle cells associated with collagen fibrils and large amounts of stromal mucosubstances. The tumor was diagnosed as MPNST (Fig. 3-D) and an immunohistochemical staining for S-100 protein also showed a strong positive reaction on the tumor cells (Fig. 3-E). The wounds healed well without any signs of recurrence during follow-up visits.
Neurofibromas are neurogenic neoplasms, which are relatively well-circumscribed, although not encapsulated, and which might originate from the peripheral nerve sheath.5 Although neurofibromas are often found in the head and neck region, their central lesions, such as in the jaw bones of the mandible or maxilla, are rare.2,5,12-14
Several subtypes of neurofibromas occur in von Recklinghausen's disease, which can be diagnosed based on gross clinical and microscopic findings. A localized neurofibroma of von Recklinghausen's disease has similar histological findings to a solitary lesion, but the von Recklinghausen's lesion can reach a larger size than a solitary one. Only 15 cases of mandibular intraosseous neurofibroma in association with neurofibromatosis have been reported in the literature.15-20 Our first two cases showing intrabony neurofibromas accompanied by cafe-au-lait spots belong to this category.
Craniofacial abnormalities in neurofibromatosis include calvarial defects, changes in the sella turcica and orbit, hypoplasia of the mandible and zygomatic arch, and abnormalities of the temporal bone. Facial or intraoral swelling is the most common clinical symptom when this tumor occurs in the jaw.5 Although the peripheral type of neurofibroma lacks encapsulation, which imposes difficulties in its complete removal, the central type in the jaw rarely poses such difficulty in its removal.3 However, the recurrence rate for solitary neurofibromas is reported to be 23.5%21 and for neurofibromas from neurofibromatosis it is reported to be 30%.2 These rates are related to the lack of encapsulation.5 All cases in this report were not completely confined to the bony cage of the mandible, and they showed the perforation or resorption of the cortices that required careful attention for the proper dissection and the selection of the appropriate margins.
Most intraosseous solitary neurofibromas are found in the mandible, probably because of the passage of nerves through the mandibular canal. They can also cause ramification of the teeth. These neurofibromas occur at various ages, from 2 to 65 years,2,20,22,23 while the soft tissue type (in the presence of von Recklinghausen's disease) occurs mostly in the younger age group (mean age, 15 years).24
The lesions are frequently accompanied by a developing dental follicle or impacted tooth. But the role of dental follicles in the pathogenesis is not clearly understood. When our two cases were histologically evaluated, the follicles were clearly separated from the main tumor tissue, which suggests that the tumor was not derived from the follicle.
On the other hand, MPNSTs in the soft tissue are uncommon, and a primary MPNST in the bone is one of the most uncommon neoplasms. Although MPNSTs are unusual in the long bones, they have been reported to occur as solitary lesions in the mandible, and they are rarely associated with neurofibromatosis.25,26 They can develop from pre-existing neurofibromas or schwannomas, de novo from the peripheral nerves, or following radiation therapy.27 The malignant transformation rate of a neurofibroma is known to be between 5-16%,2,21 and approximately 50% of MPNSTs are reported to be associated with von Recklinghausen's disease (also called NF1).2,28
Most of MPNSTs occur between 20 and 50 years of age.1,29 Interestingly, our two MPNST cases involving the jaw bones developed in teenagers without any evidence of NF1. A review of the literature on intraosseous MPNSTs by Bullock et al.25 confirmed a wider age distribution (between 4 and 76 years of age) when compared with the soft tissue counterpart,2 and none of them had a neurofibromatosis. These reports and the clinical features of our cases support the notion that primary intraosseous MPNSTs have different clinical features from those in the soft tissue.
The origin of the intraosseous MPNST is still obscure. Although it is possible that extraosseous MPNSTs invade the bony space by secondary erosion or arise from a nerve passing through a bony foramen,25 the radiologic and clinical findings of our two cases clearly show that their intraosseous origins were distant from the mandibular and mental foramen. Moreover, their periapical involvement and predominant jaw localization suggest the possibility of an odontogenic or neuroectodermal origin. However, this requires more supportive evidence.
MPNSTs show a similar histologic pattern to those of fibrosarcomas or monophasic synovial sarcoma. MPNSTs, however, have irregular contours with wavy nuclei and myxoid stroma (as shown in Cases 3 and 4), suggesting a neurogenic origin, while fibrosarcomas and monophasic synovial sarcomas show symmetrically spindled cells with fascicular patterns.9 The most widely used antigen, S-100 protein is known to be observed in 50-90% of MPNSTs cases.30 Although Leu-7 and myelin basic protein are found in 50% and 40% of them, respectively,30 none of these markers are reported to be specific for the cells of neural differentiation. Our MPNST cases showed a negative response to pan-cytokeratin and smooth muscle actin (data not shown), so we excluded the possibility of monophasic synovial sarcoma and leiomyosarcoma.
MPNSTs have been reported to be highly aggressive and have a high propensity to metastasize to distant sites.31 In addition, they tend to recur locally despite aggressive surgical approaches. A positive margin is known to be the primary and single factor for predicting a local recurrence.32,33 Therefore, treatment requires a block resection, and sometimes even radiation therapy has been recommended.34 Our cases also showed the typical ill-defined margin of MPNSTs, with an eroding pattern and the resorption of the adjacent teeth. These cases required a wide resection.
We report four cases of rare intraosseous benign and malignant peripheral nerve sheath tumors that occurred in the jaw bones, including two cases of neurofibromatosis and two cases of MPNST. Their clinical, radiographic, histopathological, and immunohistochemical characteristics were reviewed in conjunction with the age of the patient, the type of surgical intervention, and the accompanying impacted teeth in intraosseous neurofibroma. These findings suggest that intraosseous MPNSTs may have different clinical characteristics from lesions of the soft tissue.
Figures and Tables
Fig. 1
Preoperative panoramic images (A) and CT scan (B, and C) show the expansile radiolucent intraosseous lesions in the mandible (indicated by arrows in A & B) and maxilla (indicated by arrow heads in A & C). The elongated tumor cells in the interlacing bundles with surrounding collagen bundles can be seen in H-E staining (D) and they are positive for the S-100 protein (E).
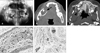
Fig. 2
Preoperative panoramic (A), CT scan (B) and dental periapical image (C) shows an ill-defined radiolucent lesion (indicated by arrows) in the mandible with root resorption of the adjacent tooth. The tumor cells do not show a palisading arrangement of the nuclei (D and E). However, there are multinucleated giant cells (indicated by arrow heads) with abnormal mitosis (indicated by arrow).
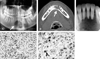
Fig. 3
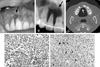
A clinical intraoral picture (A) shows a bony depression, gingival recession and root exposure (indicated by arrow) on the right maxillary incisors. A dental radiographic image (B) and maxillary CT (C) reveal an extensive osseous defect and external root resorption of the right maxillary incisors and canine. The elongated tumor cells in the interlacing bundles with surrounding collagen bundles and multinucleated giant cells with abnormal mitoses are seen in H-E staining (D) and they are positive for S-100 protein (E).
ACKNOWLEDGEMENTS
We thank Mr. Jun Chul Hong at Dept. of Oral Pathology, YUDC for providing technical support.
Notes
This work was supported from the Research Fund 2002 of College of Dentistry, Yonsei University, from the Korean health 21 research and development project by Minisitry of Health and Welfare (No. 03-PJ1-PG3-20700-0051), and from the Basic Research Program of the Korea Science & Engineering Foundation (No. R13-2003-13).
References
1. Weiss L. Cell and tissue biology; A textbook of histology. 1988. 6th ed. Munchen: Urban and Schwarzenberg;233.
2. Polak M, Polak G, Brocheriou C, Vigneul J. Solitary neurofibroma of the mandible: case report and review of the literature. J Oral Maxillofac Surg. 1989. 47:65–68.
3. Weaver BD, Graves RW, Keyes GG, Lattanzi DA. Central neurofibroma of the mandible: report of a case. J Oral Maxillofac Surg. 1991. 49:1243–1246.
4. Raghuveer HP. Solitary, central neurofibroma of the mandible: a case report. J Pierre Fauchard Acad. 1994. 8:107–109.
5. Papageorge MB, Doku HC, Lis R. Solitary neurofibroma of the mandible and infratemporal fossa in a young child. Report of a case. Oral Surg Oral Med Oral Pathol. 1992. 73:407–411.
6. Papadopoulos H, Zachariades N, Angelopoulos AP. Neurofibroma of the mandible. Review of the literature and report of a case. Int J Oral Surg. 1981. 10:293–297.
7. Griffin JE Jr, Gray CM, Schow CE Jr. Infected central neurofibroma of the mandible. Report of a case. J Oral Med. 1983. 38:94–96.
8. Langford RJ, Rippin JW. Bilateral intra-osseous neurofibromata of the mandible. Br J Oral Maxillofac Surg. 1990. 28:344–346.
9. Weiss SW, Goldblum JR. Enzinger and Weiss's soft tissue tumors. 2001. 4th ed. St. Louis: Mosby;1209–1263.
10. Hirose T, Scheithauer BW, Sano T. Perineural malignant peripheral nerve sheath tumors (MPNST): a clinicopathologic, immunohistochemical, and ultrastuctural study of seven cases. Am J Surg Pathol. 1998. 22:1368–1378.
11. Bullock MJ, Bedard YC, Bell RS, Kandel R. Intraosseous malignant peripheral nerve sheath tumor. Report of a case and review of the literature. Arch Pathol Lab Med. 1995. 119:367–370.
12. Ellis GL, Abrams AM, Melrose RJ. Intraosseous benign neural sheath neoplasms of the jaws. Report of seven new cases and review of the literature. Oral Surg Oral Med Oral Pathol. 1977. 44:731–743.
13. Chung IH, Kim NH, Choi IY. Macrodactylism associated with neurofibroma of the median nerve. A case report. Yonsei Med J. 1973. 14:49–52.
14. Ahn JY, Kwon SO, Shin MS, Shim JY, Kim OJ. A case of multiple schwannomas of the trigeminal nerves, acoustic nerves, lower cranial nerves, brachial plexuses and spinal canal: schwannomatosis or neurofibromatosis? Yonsei Med J. 2002. 43:109–113.
15. Lorson EL, DeLong PE, Osbon DB, Dolan KD. Neurofibromatosis with central neurofibroma of the mandible: review of the literature and report of case. J Oral Surg. 1977. 35:733–738.
16. Fawcett KJ, Dahlin DC. Neurilemmoma of bone. Am J Clin Pathol. 1967. 47:759–766.
17. Hunt JC, Pugh DG. Skeletal lesions in neurofibromatosis. Radiology. 1961. 76:1–20.
18. Vincent SD, Williams TP. Mandibular abnormalities in neurofibromatosis. Case report and literature review. Oral Surg Oral Med Oral Pathol. 1983. 55:253–258.
19. Thornton JB, Tomaselli CE, Rodu B, Creath CJ. Neurofibroma of the mandible in an adolescent with von Recklinghausen's disease. Pediatr Dent. 1992. 14:347–350.
20. Lee L, Yan YH, Pharoah MJ. Radiographic features of the mandible in neurofibromatosis. Oral Surg Oral Med Oral Pathol. 1996. 81:361–367.
21. Gnepp DR, Keyes GG. Central neurofibromas of the mandible: report of two cases. J Oral Surg. 1981. 39:125–127.
22. Prescott GH, White RE. Solitary, central neurofibroma of the mandible: report of case and review of the literature. J Oral Surg. 1970. 28:305–309.
23. Ellis GL, Abrams AM, Melrose RJ. Intraosseous benign neural sheath neoplasms of the jaws. Report of seven new cases and review of the literature. Oral Surg Oral Med Oral Pathol. 1977. 44:731–743.
24. Oberman HA, Sullenger G. Neurogenous tumors of the head and neck. Cancer. 1967. 20:1992–2001.
25. Bullock MJ, Bedard YC, Bell RS, Kandel R. Intraosseous malignant peripheral nerve sheath tumor. Report of a case and review of the literature. Arch Pathol Lab Med. 1995. 119:367–370.
26. Terry DG, Sauser DD, Gordon MD. Intraosseous malignant peripheral nerve sheath tumor in a patient with neurofibromatosis. Skeletal Radiol. 1998. 27:346–349.
27. Colmenero C, Rivers T, Patron M, Sierra I, Gamallo C. Maxillofacial malignant peripheral nerve sheath tumours. J Craniomaxillofac Surg. 1991. 19:40–46.
28. Dorfman HD, Czerniak B. Bone cancers. Cancer. 1995. 75:203–210.
29. Bojsen-Moller M, Myrhe-Jensen O. A consecutive series of 30 malignant schwannomas: survival in relation to clinicopathological parameters and treatment. Acta Pathol Microbiol Scand. 1977. 92A:147–158.
30. Weiss SW, Langloss JM, Enzinger FM. The role of S-100 protein in the diagnosis of soft tissue tumors with particular reference to benign and malignant Schwann cell tumors. Lab Invest. 1983. 49:299–308.
31. Dorfman HD, Czerniak B. Bone tumors. 1998. St Louis: Mosby;839–841.
32. Conley J, Janecka IP. Neurilemmoma of the head and neck. Trans Sect Otolaryngol Am Acad Opthamol Otolaryngol. 1975. 80:459–464.
33. Batsakis JG. Tumors of the head and neck; clinocopathological considerations. 1979. Baltimore: Williams and Wilkins;313–323.
34. Shafer WG, Hine MK, Levy BM. A textbook of oral pathology. 1983. Philadelphia: WB Saunders;206–208.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download