Abstract
The aim of the present study was to examine the functional changes that occur when a rabbit carotid artery is cultured in serum-free medium. In endothelium (EC)-intact arteries cultured under serum-free conditions, acetylcholine (ACh)-induced relaxation responses were partially, yet significantly, reduced when compared with freshly isolated arteries. After pretreatment with NG-nitro-L-arginine methyl ester (L-NAME), a nitric oxide synthase inhibitor, application of ACh resulted in a significant contraction in organ cultured arteries. The amplitude of the ACh-induced contractions increased with the duration of culture. In EC-denuded arteries cultured under serum-free conditions, ACh induced responses similar to those in EC-intact arteries pretreated with L-NAME. Furthermore, ACh caused a significant increase in intracellular Ca2+ concentration ([Ca2+]i) in EC-denuded arteries cultured under serum-free condition for 7 days. There was little change in either [Ca2+]i or tension in freshly isolated carotid rings. There was no difference in sodium nitroprusside-induced relaxation responses between fresh and cultured arteries. These results suggest that prolonged culture of carotid arteries under serum-free conditions changes the functional properties of vascular reactivity in rabbit carotid arteries.
Smooth muscle cells in primary culture progress from a contractile to a synthetic phenotype; a process involving changes in both morphology and the loss of contractile proteins.1 The changes in contractility have been attributed to the loss of extracellular matrix or cell-cell contact in vascular and intestinal smooth muscle primary culture.2-4 Maintenance of the contractile phenotype in vitro will allow a stable background against which interventions under controlled experimental conditions leading to phenotypic modulation (e.g. chronic control of reactivity) could be assessed.
Smooth muscle cultured under normal environmental condition (maintained extracellular matrix and cell-cell contacts) is expected to contribute to maintenance of the contractile phenotype.5,6 Indeed, studies have shown that contractility of vascular smooth muscle is well maintained in organ culture for several days, especially under serum-free conditions. in serum-free conditions, vascular cells remain in a quiescent state and smooth muscle phenotypes are well maintained, in contrast to the arteries cultured with fetal bovine serum.7-10 These reports suggest that organ culture under serum-free conditions will keep vasculature in a contractile phenotype over a longer period of time. If that is the case, it could be a useful tool for analyzing the effect of growth factors on chronic regulation of vascular tone. Rogers et al.5 reported, however, that the electrical activity of canine colonic strips was abolished after culturing strips under serum-free conditions. Furthermore, in our preliminary experiments, acetylcholine (ACh) caused vasoconstriction in organ cultured arteries, the opposite of the results obtained in freshly isolated arteries. These findings question the validity of organ culture raised under serum-free conditions as a good experimental control. Thus, the aim of the present study was to investigate phenotypic changes of endothelium or smooth muscle cells that are kept under serum-free conditions. In addition, this study attempts to determine whether the ACh-induced abnormal vascular response is associated with endothelium or smooth muscle cell organ culture conditions.
After anesthetizing a rabbit with pentobarbital sodium (60 mg/Kg), carotid arteries were isolated under sterile conditions and placed in sterile Hanks' balanced salt solution containing 1% penicillin-streptomycin. Fat and adventitia were removed and the carotid artery was cut into rings (~2 mm wide) for contraction measurements and helical strips (2 mm wide, 5 mm long) for intracellular Ca2+ concentration ([Ca2+]i) measurement. The strips were placed in 2 ml of serum-free Dulbecco's modified Eagle's medium (DMEM) with 1% penicillin-streptomycin. Strips were maintained at 37℃ in an atmosphere of 95% O2 and 5% CO2 for up to 7 days. In some experiments, the endothelium was removed by gently rubbing the intimal surface of the ring with the flat surface of a forcep. Freshly isolated arteries were also prepared as described above.
Carotid arterial rings were placed in normal physiological salt solution (PSS) that contained (mM): 136 NaCl, 5.4 KCl, 2.5 CaCl2, 1.0 MgCl2, 24 NaHCO3, 5.5 Glucose. EDTA (1 mM) was added to remove contaminating heavy metal ions which catalyze oxidation of organic chemicals in PSS. A high K+ (70 mM) solution was prepared by replacing NaCl with an equimolar amount KCl. These solutions were saturated with 95% O2 and 5% CO2 mixture at 37℃ and pH 7.4.
Muscle contraction was recorded isometrically with a force displacement transducer (UFER, Tokyo, Japan). Each arterial ring was attached to a holder under a resting tension of 0.8 g. After a 30 min equilibration in a 3 mL organ bath, each strip was repeatedly exposed to the high K+ solution until responses became stable. Concentration-response curves were obtained by cumulative application of relaxants after precontraction induced by phenylnephrine (PE) reached a steady-state level.
[Ca2+]i and the magnitude of muscle contraction were measured as described by Kwon et al.11 Briefly, muscle strips were exposed to the acethoxymethyl ester of fura-2/AM (fura-2, 5µM) in the presence of 0.01% cremophore EL for 5-6 hr at room temperature (22-24℃). After fura-2 loading, a carotid arterial strip was placed in the experimental chamber, and illuminated alternatively (48 Hz) with 340 nm and 380 nm light. The change in [Ca2+]i was expressed as the ratio of fluorescence induced by 340 nm (F340) and 380 nm (F380) light. The fluorescence was filtered using 510 nm filter and detected with a spectrophotometer (CAF 110, Japan Spectroscopic, Tokyo, Japan). The change in [Ca2+]i induced by ACh was expressed as a percentage of the PE-induced ratio.
In freshly isolated arteries (fresh arteries), acetylcholine (ACh) relaxed the phenylnephrine (PE)-induced contraction in a concentration-dependent manner. These ACh-induced relaxation responses were also observed in arteries cultured under serum-free conditions (cultured arteries). The magnitude of the ACh-induced relaxation response was significantly reduced in cultured arteries. In 3 day culture, 10-8 M ACh induced significant reduction, and 10-5-10-8 M ACh did the same in 7 day culture. To evaluate the changes in ACh-induced relaxation responses in cultured arteries, the concentration-response curve obtained after relaxation was normalized to the maximal relaxation for each experimental condition. Concentration-response curves were shifted to the right for both 3 and 7 day cultured arteries when compared with fresh arteries (Fig. 1, n = 14).
When arteries were pretreated with L-NAME (3 × 10-5 M), a Nitric Oxide Synthase (eNOS) inhibitor, ACh-induced relaxation was partially yet significantly reduced in fresh arteries. Peak relaxations occured at 10-5 M ACh and were 91.8 ± 2.7% vs. 23.5 ± 4.2% (control vs. L-NAME treated fresh arteries, n = 12). In cultured arteries, ACh-induced relaxation was completely abolished at low concentration, however, a high concentration of ACh caused a contraction that correlated with the duration of culture. The peak contractions occurred at 10-5 M ACh and were 13.0 ± 3.4% and 35.2 ± 5.3% of PE-induced contraction in 3 days and 7 days cultured arteries, respectively (n = 10, Fig. 2).
To investigate the involvement of endothelium (EC) on ACh-induced contraction in cultured arteries, we measured the effect ACh was on vascular contractility in EC-denuded arteries. In EC-denuded, fresh arteries, cumulative application of ACh had no apparent effect on tension (peak contraction at 10-5 M ACh; 2.6 ± 0.8%; n = 14). In EC-denuded, cultured arteries, cumulative application of ACh caused a significant increase in tension that correlated to the duration of culture. The peak contractions occured at 10-5 M ACh and were 7.1 ± 2.7% and 32.2 ± 7.9% in 3 and 7 day cultured arteries, (n = 10, Fig. 3). There was no statistical difference in the amount of ACh-induced contraction between EC-intact and EC-denuded cultured arteries when treated with L-NAME. In L-NAME-treated 7 day cultured arteries at the peak of 10-5 M ACh, contractions of 35.2 ± 5.3% vs. 32.2 ± 7.9% occurred in EC-intact vs. EC-denuded, respectively (p > 0.05, n = 10).
We also examined the relaxant effect of sodium nitroprusside (SNP) on the PE-induced contraction. In both EC-denuded fresh and cultured arteries, SNP relaxed the PE-induced precontraction (1 µM PE) in a concentration-dependent manner (0.001 to 10 µM SNP). The relaxant effects of SNP in the fresh and cultured arteries were not significantly different. Peak relaxations of 105.6 ± 1.7% vs. 103.4 ± 1.0% occurred at 10-5 M SNP in fresh vs. cultured arteries (p > 0.05, n = 15, Fig. 4).
To investigate the mechanism of ACh-induced contraction in cultured arteries, we measured the [Ca2+]i and contraction simultaneously in EC-denuded and L-NAME pretreated arteries. In EC-denuded, fresh arteries, 10 µM ACh had no effect on [Ca2+]i and contraction inducing -4.0 ± 1.6% and 5.0 ± 1.6% changes in [Ca2+]i and contraction, respectively (n = 6). But in EC-denuded, cultured arteries, ACh (10 µM) caused a significant increase both in contraction and [Ca2+]i. The percent changes in [Ca2+]i and contraction were 20.4 ± 5.2% and 34.9 ± 7.4%, respectively (n = 6). The differences between fresh and cultured arteries for both contraction and [Ca2+]i are significant (Fig. 5).
The results show that ACh-induced EC-dependent relaxation (EDR) in cultured arteries raised in serum-free medium was significantly reduced. Also, ACh caused significant contraction in cultured arteries pretreated with L-NAME. Agonists like ACh produce nitric oxide (NO) by increasing [Ca2+] in endothelium thus activating eNOS.12,13 NO produced by EC then activates guanylate cyclase and increases cGMP content in smooth muscle.14 cGMP activates cGMP-dependent protein kinase, which leads to muscle relaxation by decreasing [Ca2+] and/or the Ca2+ sensitivity of the contractile apparatus.15 If ACh-induced EDR in cultured arteries is reduced due to decreased production of NO, pretreatment with L-NAME should abolish the ACh-induced EDR difference between fresh and cultured arteries. It is interesting, however, that ACh-induced EDR was partly inhibited by L-NAME in fresh arteries, yet ACh caused vasoconstriction in cultured arteries (Fig. 2). Furthermore, an increase in eNOS mRNA and upregulation of NO production in mesenteric arteries cultured under serum-free conditions have been reported.8 Another possible explanation is that NO responsiveness may be decreased in cultured smooth muscle arteries. In the present study, though, there was no difference in SNP-induced relaxation responses between fresh and cultured arteries (Fig. 4). These results suggest that a reduction of the ACh-induced EDR in the present experiment might be due to enhanced production of vasoconstrictive substance (eg, endothelin) in endodermal tissue or the changes in the functional properties of smooth muscle in cultured arteries. The overproduction of vasoconstrictive agents can be ruled out, because we found similar ACh-induced vasoconstriction in EC-denuded arteries (Fig. 3).
The contractility of smooth muscle depends on [Ca2+] or the Ca2+ sensitivity of the contractile element. The level of [Ca2+] can be increased by Ca2+ influx from extracellular space or release from internal stores.16 ACh can increase the [Ca2+] through activation of muscarinic receptors (M2 or M3 receptors), which results in Ca2+ release from internal stores.17 The application of ACh in EC-denuded arteries increased [Ca2+] in cultured arteries, but not in fresh arteries. This ACh-induced Ca2+ increase might be due to over-expression of muscarinic receptors or supersensitivity to ACh. In a study with rat tail arteries, Lindqvist et al18 reported that sensitivity to norepinephrine was higher in organ cultured arteries as compared to freshly isolated arteries. Abel et al.19 also observed sensitization to norepinephrine in organ cultured portal veins. Rogers et al.5 reported a sensitization to ACh in organ cultured canine colonic smooth muscle. These reports suggest the possibility that sensitivity to ACh or over-expression of muscarinic receptors may occur in rabbit carotid arteries during organ culture under serum-free conditions, and result in an increase in [Ca2+] and tension in cultured arteries.
In summary, EC-dependent relaxation induced by ACh was partially but significantly reduced in cultured arteries. After pretreatment of L-NAME, ACh caused significant vasoconstriction in cultured arteries, and increased both tension and [Ca2+] in EC-denuded, cultured arteries. The SNP-induced relaxation response was not changed with culture. These results may provide insight into how the functional changes of vessel tissue occur during organ culture.
Figures and Tables
 | Fig. 1Concentration-response relationship for the relaxant effect of acetylcholine (ACh) on the phenylnephrine (PE)-induced contractions in endothelium (EC)-intact rabbit carotid arteries. Carotid arterial rings were cultured in serum-free Dulbecco's modified Eagle's medium (DMEM) for up to 7 days. ACh was cumulatively added after PE-induced contraction had reached a steady state. The difference between steady state precontraction and basal tension was considered one hundred percent. Results are expressed as mean ± S.E. (n = 14). The inset indicates the concentration-relaxation relationship after the relaxation was normalized to the maximal relaxation at each experimental condition. *denotes significant difference from freshly isolated arteries (p < 0.01). |
 | Fig. 2Effect of Nitric oxide synthase inhibitor, L-NAME, on the ACh-induced relaxation responses in EC-intact, freshly isolated rabbit carotid arteries (A) and arteries cultured in serum-free DMEM for up to 7 days (B). Concentration-response curve shows ACh-induced change of tension in arteries that were: freshly prepared (○), cultured for 3 days (●), and cultured for 7 days (▲). NG-nitro-L-arginine methyl ester (L-NAME; 30 µM) was added 30 minutes before PE (1 µM) application. Results are expressed as mean ± S.E. (n = 10). *and **denote significant difference from freshly isolated arteries (p < 0.05 and p < 0.01, respectively). |
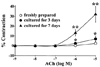 | Fig. 3Concentration-response relationship of the effect of ACh on EC-denuded carotid arteries that were freshly isolated (●),cultured in serum free DMEM for 3 days (○) or cultured for 7 days (▲). ACh was cumulatively added after PE-induced contraction had reached a steady state. The difference between steady state precontraction and basal tension was considered one hundred percent. Results are expressed as mean ± S.E. (n = 10). *and **denote significant difference from freshly prepared arteries (p < 0.05 and p < 0.01, respectively). |
 | Fig. 4Concentration-response relationship of the effect of SNP on endothelium-denuded carotid arteries that were freshly isolated (●), cultured for 3 days (○) and 7 days (▲). Sodium nitroprusside (SNP) was cumulatively added after PE-induced contraction had reached a steady state. The difference between steady state precontraction and basal tension was considered one hundred percent. Results are expressed as mean ± S.E. (n = 15). |
 | Fig. 5ACh-induced changes in [Ca2+]i and contraction in EC-denuded, freshly prepared arteries and carotid arterial strips cultured in serum-free DMEM for 7 days. A) Typical recordings of simultaneous measurement of [Ca2+]i (upper panel) and tension (lower panel) in rabbit carotid arteries. L-NAME (30 µM) was added 30 minutes before application of PE (1 µM). ACh (10 µM) was added after PE-induced contraction reached steady-state conditions. B) Mean changes in [Ca2+]i induced by ACh in freshly prepared arteries and arteries cultured for 7 days. Results are expressed as mean ± S .E. (n = 6). The PE-induced change in ratio was considered one hundred percent. C) Mean changes in contraction induced by ACh in freshly prepared arteries and arteries cultured for 7 days. Results are expressed as mean ± S.E. (n = ). The PE-induced contraction was considered one hundred percent. *denotes significant difference from freshly prepared arteries (p < 0.01). |
References
1. Thyberg J, Nilsson J, Palmberg L, Sjolund M. Adult human arterial smooth muscle cells in primary culture. Modulation from contractile to synthetic phenotype. Cell Tissue Res. 1985. 239:69–74.
2. Chamley-Campbell JH, Campbell GR, Ross R. The smooth muscle cell in culture. Physiol Rev. 1979. 59:1–61.
3. Davies PF. Vascular cell interactions with special reference to the pathogenesis of atherosclerosis. Lab Invest. 1986. 55:5–24.
4. Stadler E, Campbell JH, Campbell GR. Do cultured vascular smooth muscle cells resemble those of the artery wall? If not, why not? J Cardiovasc Pharmacol. 1989. 14:Suppl 6. S1–S8.
5. Rogers MJ, Ward SM, Horner MA, Sanders KM, Horowitz B. Characterization of the properties of canine colonic smooth muscle in culture. Am J Physiol. 1993. 265(5 pt 1):C1433–C1442.
6. Bakker ENTP, Van der Meulen ET, Spaan JAE, Vanbavel ED. Organoid culture of cannulated rat resistance arteries: effect of serum factors on vasoactivity and remodeling. Am J Physiol Heart Circ Physiol. 2000. 278:H1233–H1240.
7. Lindqvist A, Nilsson BO, Hellstrand P. Inhibition of calcium entry preserves contractility of arterial smooth muscle in culture. J Vasc Res. 1997. 34:103–108.
8. Yamawaki H, Sato K, Hori M, Ozaki H, Nakamura S, Nakayama H, et al. Impairment of EDR by a long-term PDGF treatment in organ-cultured rabbit mesenteric artery. Am J Physiol. 1999. 277:H318–H323.
9. Yamawaki H, Sato K, Hori M, Ozaki H, Nakamura S, Nakayama H, et al. Morphological and functional changes of rabbit mesenteric artery cultured with fetal bovine serum. Life Sci. 2000. 67:807–820.
10. Murata T, Suzuki N, Yamawaki H, Sato K, Hori M, Karaki H, et al. Dexamethasone prevents impairment of endothelium-dependent relaxation in arteries cultured with fetal bovine serum. Eur J Pharmacol. 2005. 515:134–141.
11. Kwon SC, Park KY, Ahn DS, Lee HY, Kang BS. The effect of NO donor on contraction, cytosolic Ca2+ level and ionic currents in guinea pig ileal smooth muscle. Korean J Physiol Pharmacol. 2000. 4:33–40.
12. Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980. 288:373–376.
13. Gryglewski RJ, Botting RM, Vane JR. Mediators produced by the endothelial cell. Hypertension. 1988. 12:530–548.
14. Rapoport RM, Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983. 52:352–357.
15. Karaki H, Sato K, Ozaki H, Murakami K. Effect of sodium nitroprusside on cytosolic calcium level in vascular smooth muscle. Eur J Pharmacol. 1988. 156:259–266.
16. Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990. 259(1 Pt 1):C3–C18.
17. Eglen RM, Reddy H, Watson N, Challiss RA. Muscarinic acetylcholine receptor subtypes in smooth muscle. Trends Pharmacol Sci. 1994. 15:114–119.
18. Lindqvist A, Nordstrom I, Malmqvist U, Nordenfelt P, Hellstrand P. Long-term effects of Ca2+ on structure and contractility of vascular smooth muscle. Am J Physiol. 1999. 277(1 Pt 1):C64–C73.
19. Abel PW, Trapani A, Aprigliano O, Hermsmeyer K. Trophic effect of norepinephrine on rat portal vein in organ culture. Circ Res. 1980. 47:770–775.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download