Abstract
The aims of this study were to identify the morphological diversities and anatomical variations of pancreatic ductal system and to define the relationships between pancreatic ductal systems, pancreaticobiliary diseases, and procedure-related complications, including post-ERCP pancreatitis. This study included 582 patients in whom both pancreatic duct (PD) and common bile duct were clearly visible by ERCP. PD systems were categorized into four types according to the relationship between common bile duct and PD. In types A and B, Wirsung duct formed the main PD. In type C, Wirsung duct did not form the main PD. If PD system did not fall into any of these three types, it was categorized as type D. The distribution of types among pancreatic ducts examined was as follows: type A: 491 cases (84.4%), type B: 56 cases (9.6%), type C: 20 cases (3.4%), and type D: 15 cases (2.6%). The anomalous anatomic variations of PD systems were divided into migration, fusion, and duplication anomalies. PD anomalies were noted in 51 patients, of which 19 (3.3%) were fusion anomalies (12 complete pancreas divisum, 7 incomplete pancreas divisum), and 32 (5.5%) were duplication anomalies (5 number variations, 27 form variations). No significant relationships between various PD morphologies and pancreaticobiliary diseases were found. However, post-ERCP hyperamylasemia was more frequently found in types C (41.7%), D (50%) and A (19.8%) than in type B (9.4%). In summary, whether Wirsung duct forms the main PD and the presence or absence of the opening of the Santorini duct are both important factors in determining the development of pancreatitis and hyperamylasemia after ERCP.
Endoscopic retrograde cholangiopancreaticography (ERCP) allows for visualization of pancreatic duct (PD) and bile duct. It is the confirmative tool for diagnosis and treatment of disorders of PD and bile duct, even though magnetic resonance cholangiopancreaticography has emerged as an alternative procedure.1-5 In addition, the images of PD and bile duct obtained by ERCP have been shown to agree with the pattern and anomaly of PD detected by surgery or autopsy.6,7
Due to the close relationship between pancreatic ductal system and bile duct in development and anatomy, abnormalities in one often affect the other. For example, the anomalous pancreaticobiliary ductal union is one of the important risk factors for gallbladder and bile duct cancer.8,9 However, the relationship between the morphology of PD and pancreaticobiliary diseases has not been clearly elucidated. Hence, based upon the images of pancreatic ductal system and bile duct obtained by ERCP, patterns of PD and anatomical variations were classified, and associations between the patterns and anomalies with pancreaticobiliary diseases and post-ERCP complications were assessed.
Among patients who underwent ERCP at Severance Hospital, Yonsei University College of Medicine from January 1992 to November 1996, 582 patients whose bile duct and PD were completely visualized were included in this study. The patients' medical records and radiologic images, including the ERCP image, were retrospectively reviewed. ERCP was performed according to standard procedures, with the patient under sedation with intramuscular meperidine (up to 1 mg/kg, but no more than 50 mg) and midazolam.
Based on the relationship of pancreatic Wirsung and Santorini ducts and common bile duct to each other and to duodenum, the ductal systems were divided into four groups as follows (Fig. 1): In type A and B, Wirsung duct is the main PD and it drains into the major papilla of duodenum. In type A, Santorini duct is an accessory PD which is obliterated or absent (if the accessory PD is absent, the main PD should be straight rather than curved like a chain or wheel). Santorini duct of type B is an accessory PD and runs into the minor papilla of duodenum. The type C is a PD system in which Santorini duct forms the main PD and drains into the minor papilla of duodenum. Wirsung duct of type C forms an accessory PD which is obliterated or drains into the major papilla of duodenum. The PD systems other than type A, B, and C are were defined as type D. In addition, according to the morphology of the main PD and the accessory PD, type A was divided into 6 subtypes, type B into 5 subtypes, and types C and D were divided into 4 subtypes each (Fig. 1).10
Anatomical anomalies of PD were classified as either a migration anomaly (annular pancreas, ectopic pancreas), a fusion anomaly (complete, incomplete pancreas divisum), or a duplication anomaly (number variation, form variation) according to developmental aspects of PD system.11
The definition of pancreatitis and the grading of its severity were based on consensus criteria.12 Post-ERCP pancreatitis was diagnosed when new-onset or increased abdominal pain lasted for more than 24 hours, caused the unplanned admission of an outpatient for more than one night, or prolonged the planned admission of an inpatient, and was associated with a serum amylase level increase of at least three times above normal, at approximately 18 hours (the next morning) after the procedure. Post-ERCP hyperamylasemia was defined as a normal clinical condition at 24 h after the procedure but with serum amylase levels elevated above the normal upper limit (115 U/L).
The operators rated selective cannulation as "difficult" when three or more attempts were required, due to difficulty in reaching the proper placement angle for selective cannulation, unusual anatomy, and/or accidental submucosal contrast media injection. If bile duct cannulation was difficult, a needle knife or precut papillotomy was performed, and/or a guide wire through the second sphincterotome or standard catheter channel was used, or the procedure was abandoned and repeated at a later session.
The mean age of the study population (n = 582) was 52.8 years. Three hundred twenty-five of the patients were male. The indications for ERCP included choledocholithiasis, jaundice, chronic pancreatitis, pancreatic tumor, abdominal pain, and bile duct dilatation. The final diagnosis, confirmed with ERCP, was gallstone in 253 cases (40.4%), chronic pancreatitis in 77 cases (13.2%), cholangiocarcinoma in 48 cases (8.2%), pancreatic cancer in 39 cases (6.7%), and acute pancreatitis in 21 cases (3.6%).
The frequency of PD types found was as follows: type A: 491 cases (84.4%), type B: 56 cases (9.6%), type C: 20 cases (3.4%), and type D: 15 cases (2.6%). The type B system, in which Santorini duct is present and drains into the minor papilla of duodenum, was found more frequently in men (11.7% in men vs. 1.5% in women). There were no other significant differences between genders. The main types were further subdivided according to the morphologic relationship between the main and accessory ducts. The number of cases seen for each type was as follows: subtype A1: 254 cases (43.6%), subtype A2: 70 cases (12%), subtype A3: 15 cases (2.6%), subtype A4: 2 cases (0.3%), subtype A5: 26 cases (4.5%), and subtype A6: 124 cases (21.3%); subtype B1: 37 cases (6.4%), B2: 6 cases (1.0%), B3: 10 cases (1.7%), B4: 2 cases (0.3%) and B5: 1 case (0.2%); subtype C1: 12 cases (2.1%), C2: 7 cases (1.2%), C4: 1 case (0.2%), and C3 subtype was not detected; subtype D1: 12 cases (2.0%), and D2, D3, and D4 had 1 case (0.2%) each (Table 1).
An anatomical anomaly of the pancreatic duct was observed in a total of 51 cases (8.8%). A fusion anomaly was found in 19 cases (3.3%), and a duplication anomaly was found in 32 cases (5.5%). In the fusion anomaly cases, a complete pancreatic divisum was seen in 12 cases (2.1%) and an incomplete pancreatic divisum was seen in 7 cases (1.2%). In the duplication anomaly cases, number variation was seen in 5 cases (0.9%) and form variation in 27 cases (4.6%) with ring-type in 18 cases (3.1%), and spiral-type in 9 cases (1.5%) (Table 2).
No statistically significant correlation was detected between PD type and pancreatic-bile duct diseases, including choledocholithiasis, cholangiocarcinoma, acute and chronic pancreatitis, and pancreatic cancer (Table 3).
However, the relationship between the type of PD system and post-ERCP complications was different. The assessment of the presence of asymptomatic hyperamylasemia and pancreatitis after ERCP was possible in 289 of 582 patients. Among these patients, asymptomatic hyperamylasemia occurred in 59 cases (20.4%). The analysis of the post-ERCP hyperamylasemia according to PD type showed that the rate of post-ERCP hyperamylasemia was 19.8% (47/237) in type A, 9.4% (3/32) in type B, 41.7% (5/12) in type C, and 50% (4/8) in type D cases (Table 4). The rate of hyperamylasemia in types C and D was significantly higher than in types A and B (p = 0.018). Clinically overt post-ERCP pancreatitis occurred in 1.7% (5/289) of the evaluated patients. There were no significant differences between the four PD types for other factors related to post-ERCP pancreatitis or hyperamylasemia, including age, gender, and difficulty in visualization of pancreaticobiliary ductal system.
Pancreatic ductal system, as well as the closely related bile duct, shows diverse morphology and anatomical variation, and various classification methods have been reported.13-19 However, with these methods, a detailed classification based on the morphology of pancreatic duct is not possible. In current study, the morphology of pancreatic duct was evaluated based on the classification system described by Stolte et al.10 In this system, pancreatic ductal systems are classified into four major types according to the relationship between Wirsung duct, Santorini duct, and common bile duct. The types were then further subdivided based on the morphology of Wirsung duct and Santorini duct, and thus provided a more precise classification.
The results of this study are similar to those of previous reports. The type in which Wirsung duct forms the main pancreatic duct and opens into the major papilla turned out to be the most prevalent, and the frequency of this type was approximately 66-90% in previous studies.15,16 Santorini duct has been reported to be present in 50-100% of cases in previous studies,7 and in this study the frequency was 76.7%. Even though the B type tended to be more prevalent in males and D type more prevalent in females, there was no significant difference in the morphology of pancreatic ductal system between genders.
The anatomical anomalies of pancreatic duct were classified based on the developmental system proposed by Siegel et al.11 In this study, migration anomalies such as annular pancreas and ectopic pancreas were not detected. This is partially attributable to a low incidence of annular pancreas, about 3 in 20,000 cases of autopsies,17 and to technical difficulty in the detection of ectopic pancreas with ERCP. Fusion anomalies are sub-classified into complete pancreas divisum and incomplete pancreas divisum. Complete pancreas divisum has been reported as a relatively rare condition with the frequency of 0.5-11%, and in our study, the frequency was 2.1%.16,20 Incomplete pancreas divisum is less frequent than complete pancreas divisum, the frequency is approximately 0.13-0.9%, and in our study, it was 1.2%, which is slightly higher than previous reports.21,22 Duplication anomalies are also uncommon, and Uomo et al.23 reported the frequency to be approximately 4.6%, which is similar to the 5.5% detected in our study.
Acute pancreatitis, chronic pancreatitis, and pancreatic cancer were more prevalent in type C ductal systems than in other types, however, a statistically significant correlation was not detected. The type C pancreatic ductal system contains the fusion anomalies such as complete or incomplete pancreas divisum. Although further study is warranted, the complete pancreas divisum is reported as associated with acute and recurrent pancreatitis.23-28 The frequency of complete pancreas divisum in the cases of acute pancreatitis is reported as approximately 25-38%.23,27 In our study, complete pancreas divisum associated with acute pancreatitis was more frequent than the cases diagnosed without concurrent acute pancreatitis (9.5% vs. 2.0%).
No statistically significant correlation was found when examining the association between bile duct diseases and the morphology of the pancreatic duct. However, this lack of correlation may not indicate that there is never a correlation because the classification system adopted in this study is oriented toward pancreatic ductal morphology rather than the relationship of the pancreaticobiliary ductal system.
The frequencies of post-ERCP pancreatitis and asymptomatic hyperamylasemia were 1.7% and 20.4%, respectively. No significant complications, including perforation, hemorrhage or severe pancreatitis, were seen in this study. Pancreatic duct type was the only risk factor found in this study for post-ERCP hyperamylasemia and pancreatitis, though this may be associated with selection bias. This study included cases in which both pancreatic duct and biliary duct were completely visualized. With this criterion, some difficult cases in the ERCP procedure might be excluded.
The rate of post-ERCP pancreatitis in this study (1.7%) is comparable to previous studies in which it was 1.3-11.3%.29-31 As post-ERCP pancreatitis was less frequent in types A and B, it is likely related to whether or not Wirsung duct forms the main pancreatic duct.
On the contrary, the incidence of post-ERCP hyperamylasemia in this study was 20.4%, which is slightly higher than that reported in previous studies (7.7-16.5%).32-34 Considering that the incidence of post-ERCP hyperamylasemia in type A cases was higher than in type B cases, the presence of Santorini duct may have an influence on the risk of post-ERCP hyperamylasemia and pancreatitis. Although asymptomatic hyperamylasemia is usually irrelevant to clinical prognosis, it has been reported that some cases with hyperamylasemia show a temporal pancreatic endocrine dysfunction, such as glucose intolerance.35 Thus, asymptomatic hyperamylasemia may reflect damage to the pancreas by ERCP.
In conclusion, differences in the morphology of pancreatic ductal system seem to be unrelated to demographic factors including gender and pancreaticobiliary diseases, although complete pancreas divisum may be associated with acute pancreatitis. However, there are possible relationships between the morphologic type of the pancreatic ductal system and post-ERCP hyperamylasemia and pancreatitis. Whether or not Wirsung duct forms the main pancreatic duct is an important factor in determining the development of pancreatitis after ERCP. When visualizing pancreatic ductal system during the ERCP, this relationship should be taken into consideration.
Figures and Tables
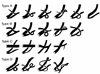 | Fig. 1Classification of Pancreatic Ductal System. In types A and B, Wirsung duct formed the main PD, however, in type C, Wirsung duct did not form the main PD. In type A, Santorini duct is either obliterated or absent. Santorini duct of type B is an accessory PD and runs into the minor papilla of duodenum. If PD system did not fall into any of these 3 groups, it was classified as type D. |
References
1. Oi I, Kobayashi S, Kondo T. Endoscopic pancreatocholangiography. Endoscopy. 1970. 2:103–106.
2. Griffin N, Wastle ML, Dunn WK, Ryder SD, Beckingham IJ. Magnetic resonance cholangiopancreatography versus endoscopic retrograde cholangiopancreatography in the diagnosis of choledocholithiasis. Eur J Gastroenterol Hepatol. 2003. 15:809–813.
3. Talwalkar JA, Angulo P, Johnson CD, Petersen BT, Lindor KD. Cost-minimization analysis of MRC versus ERCP for the diagnosis of primary sclerosing cholangitis. Hepatology. 2004. 40:39–45.
4. Hunerbein M, Stroszczynski C, Ulmer C, Handke T, Felix R, Schlag PM. Prospective comparison of transcutaneous 3-dimensional US cholangiography, magnetic resonance cholangiography, and direct cholangiography in the evaluation of malignant biliary obstruction. Gastrointest Endosc. 2003. 58:853–858.
5. Heiss FW, Shea JA. Association of pancreatitis and variant ductal anatomy: dominant drainage of the duct of Santorini. Am J Gastroenterol. 1978. 70:158–162.
6. Phillip J, Koch H, Classen M. Variations and anomalies of the papilla of Vater, the pancreas and the biliary duct system. Endoscopy. 1974. 6:70–77.
7. Belber JP, Bill K. Fusion anomalies of the pancreatic ductal system: differentiation from pathologic states. Radiology. 1977. 123:637–642.
8. Jung YS, Lee KJ, Kim H, Kim WH, Kim IG, Yoo BM, et al. Risk factor for extrahepatic bile duct cancer in patients with anomalous pancreaticobiliary ductal union. Hepatogastroenterology. 2004. 51:946–949.
9. Chang LY, Wang HP, Wu MS, Huang HT, Wang HH, Lin CC, et al. Anomalous pancreaticobiliary ductal union- an etiologic association of gallbladder cancer and adenomyomatosis. Hepatogastroenterology. 1998. 45:2016–2019.
10. Cubilla AL, Fitzgerald PJ. Cubilla AL, Fitzgerald PJ, editors. Gross anatomy. Tumors of the exocrine pancreas. 1984. Washington, DC: Armed forces institute of pathology;31–52. 2nd series, Fascicle 19.
11. Siegel HJ. Siegel HJ, editor. Radiologic interpretation: Normal biliary system and variations; normal pancreatic duct and variations. Endoscopic retrograde cholangiopancreatography: technique, diagnosis and therapy. 1992. New York: Raven Press Ltd;41–59.
12. Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991. 37:383–393.
13. Millbourn E. On the excretory ducts of the pancreas in man, with special reference to their relations to each other, to the common bile duct and to the duodenum. A radiological and anatomical study. Acta Anat. 1950. 9:1–34.
14. Dawson W, Langman J. An anatomical-radiological study on the pancreatic duct pattern in man. Anat Rec. 1961. 139:59–68.
15. Kleitsch WP. Anatomy of the pancreas. Arch Surg. 1955. 71:795–802.
16. Kozu T, Suda K, Toki F. Pancreatic development and anatomical variation. Gastrointest Endosc Clin N Am. 1995. 5:1–30.
17. Ravitch MM, Woods AC Jr. Annular pancreas. Ann Surg. 1950. 132:1116–1127.
18. Sigfusson BF, Wehlin L, Lindstrom CG. Variants of pancreatic duct system of importance in endoscopic retrograde cholangiopancreatography. Observations on autopsy specimens. Acta Radiol Diagn (Stockh). 1983. 24:113–128.
19. Yatto RP, Siegel JH. Variant pancreatography. Am J Gastroenterol. 1983. 78:115–118.
20. Carr-Locke DL. Pancreas divisum. the controversy goes on? Endoscopy. 1991. 23:88–90.
21. Sugawa C, Walt AJ, Nunez DC, Masuyama H. Pancreas divisum: is it a normal anatomic variant? Am J Surg. 1987. 153:62–67.
22. Tulassay Z, Papp J, Farkas IE. Diagnostic aspects of incomplete pancreas divisum. Gastrointest Endosc. 1986. 32:428.
23. Uomo G, Manes G, D'Anna L, Laccetti M, Di Gaeta S, Rabitti PG. Fusion and duplication variants of pancreatic duct system. Clinical and pancreatographic evaluation. Int J Pancreatol. 1995. 17:23–28.
24. Burtin P, Person B, Charneau J, Boyer J. Pancreas divisum and pancreatitis: a coincidental association? Endoscopy. 1991. 23:55–58.
25. Delhaye M, Cremer M. Clinical significance of pancreas divisum. Acta Gastroenterol Belg. 1992. 55:306–313.
26. Gregg JA. Pancreas divisum: its association with pancreatitis. Am J Surg. 1977. 134:539–543.
27. Cotton PB. Congenital anomaly of pancreas divisum as cause of obstructive pain and pancreatitis. Gut. 1980. 21:105–114.
28. Bernard JP, Sahel J, Giovannini M, Sarles H. Pancreas divisum is a probable cause of acute pancreatitis: a report of 137 cases. Pancreas. 1990. 5:248–254.
29. Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, De Berardinis F, et al. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998. 48:1–10.
30. LaFerla G, Gordon S, Archibald M, Murray WR. Hyperamylasaemia and acute pancreatitis following endoscopic retrograde cholangiopancreatography. Pancreas. 1986. 1:160–163.
31. Johnson GK, Geenen JE, Johanson JF, Sherman S, Hogan WJ, Cass O. Evaluation of post-ERCP pancreatitis: potential causes noted during controlled study of differing contrast media. Midwest Pancreaticobiliary Study Group. Gastrointest Endosc. 1997. 46:217–222.
32. Christoforidis E, Goulimaris I, Kanellos I, Tsalis K, Demetriades C, Betsis D. Post-ERCP pancreatitis and hyperamylasemia: patient-related and operative risk factors. Endoscopy. 2002. 34:286–292.
33. Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996. 335:909–918.
34. Dickinson RJ, Davies S. Post-ERCP pancreatitis and hyperamylasaemia: the role of operative and patient factors. Eur J Gastroenterol Hepatol. 1998. 10:423–428.
35. Tulassay Z, Papp J, Koranyi L, Szathmari M, Tamas G Jr. Hormonal and biochemical changes following endoscopic retrograde cholangio-pancreatography. Acta Gastroenterol Belg. 1981. 44:538–544.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download