Abstract
Lipopolysaccharide (LPS), given in vivo, modulates opossum esophageal motor functions by inducing the inducible nitric oxide synthase (iNOS), which increases nitric oxide (NO) production. Superoxide, a NO scavenger, is generated during this endotoxemia. Superoxide is cleared by superoxide dismutase (SOD) and catalase (CAT) to protect the physiological function of NO. This study examined whether lower esophageal sphincter (LES) motility, NO release, and iNOS and nitrotyrosine accumulation in the LES are affected by LPS in vitro. Muscle strips from the opossum LES were placed in tissue baths containing oxygenated Krebs buffer. NO release was measured with a chemiluminescence NOx analyzer, and Western blots were performed to analyze iNOS and nitrotyrosine production. The percent change in resting LES tone after a 6-hour exposure to LPS was significantly increased compared to pretreatment values. The percent LES relaxation upon electrical stimulation was significantly decreased in the control group at 6 hours, indicating that the LPS treatment had an effect. The NO concentration in the tissue bath of LPS-treated muscle without nerve stimulation was significantly less than that of LPS treatment combined with SOD/CAT or SOD/CAT alone. iNOS and nitrotyrosine were detectable and increased over time in the LES muscle of both the control and LPS-treated groups. Antioxidant enzymes may play a role in regulating NO-mediated neuromuscular functions in the LES.
Previous studies have demonstrated that endotoxins induce the inducible nitric oxide synthase (iNOS), which produces large amounts of nitric oxide (NO) and in turn, alters esophageal motor function.1,2 Lipopolysaccharide (LPS) treatment results in an increase in the plasma nitrite/nitrate ratio, an index of NO generation, and this increase can be attenuated by aminoguanidine (AG), a selective iNOS inhibitor. Western blots from lower esophageal sphincter (LES) tissues demonstrate that iNOS expression is markedly increased by LPS, and AG attenuates this increase.2 Thus, LPS given in vivo modulates opossum esophageal motor functions by inducing iNOS, which increases NO production.
NO-mediated relaxation of LES is reduced by the presence of superoxide.4 The superoxide anion, a scavenger of NO, is produced in excess as part of inflammatory processes, including endotoxemia,3 and reacts rapidly with NO to form peroxynitrite (ONOO-). The antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT) prolong the biologic activity of NO through prevention of its conversion to peroxynitrite by clearing superoxide.5 However, there have been no studies on the local and direct effects of LPS and antioxidant enzymes on LES muscle function and NO generation to document their direct relationships. The aim of this study was, therefore, to investigate whether LES motility, NO release, iNOS and nitrotyrosine accumulation, a footprint of peroxynitrite, in the LES are affected by LPS with or without the presence of antioxidant enzymes in vitro.
All procedures, care of animals and conduct of experiments were carried out according to protocols from the Iowa City Veterans Affairs and the University of Iowa Animal Care and Use Committees. Fasted adult opossums of either sex weighed 2-4 kg.
Opossums were anesthetized with ketamine HCl (30 mg/kg)/acepromazine (0.3 mg/kg) given intramuscularly and sodium pentobarbital (50 mg/kg) given intraperitoneally. The chest and abdomen were opened, and the entire intrathoracic and intraabdominal esophagus was marked and measured in situ. The esophagus was transected at the proximal mark and was excised along with a cuff of gastric tissue. The entire tissue was opened in the long axis of the esophagus along the lesser curve of the stomach. The tissue was washed with warmed, aerated Krebs solution and pinned flat in a tissue bath at its dimensions in situ with the mucosal surface facing up. The bath contained Krebs solution that was maintained at 37℃ and bubbled with 95% O2-5% CO2.
The mucosa and submucosa were removed by sharp dissection. The circular muscle of the LES was identified by gross inspection. One transverse muscle strip measuring 2 mm in width and 1 cm in length was cut from the LES so that the long axis of the muscle strip paralleled the long axis of the smooth muscle cells comprising the circular muscle layer. One end of the strip was fixed to a glass hook and the other was attached by a surgical silk to a Radnoti isometric force-displacement transducer. The transducer was mounted on a rack-and-pinion assembly so that the length of the muscle strip was between 2 platinum wire electrodes that ran parallel to the muscle strip at a distance of 4 mm from one another. The muscle strip and electrode assembly were immersed in a 5 cc jacketed tissue bath that contained Krebs solution that was maintained at 37℃ and aerated with 95% O2-5% CO2. The output of the forcedisplacement transducer was displayed and recorded on a Macintosh II Ci computer equipped with a Mac Lab multichannel recorder. The muscle strips were stretched rapidly until 100 mg of sustained tension was generated. This was considered the initial length. The strips were equilibrated for one hour in the warmed, oxygenated Krebs solution prior to experimentation. Changes in the resting tension of the LES were measured before and after control (Krebs solution), LPS (1 µg/mL), or LPS with Cu, Zn-SOD (25 or 100 units/mL) treatments for 6 hours.
The stimulating electrodes were attached to the output of a Grass S11 stimulator (Grass Instrument Company, Quincy, MA, USA), which delivered 4 sec trains of 1 msec, 35 V square wave pulses at 3 Hz. Only muscle strips showing reproducible responses to electrical field stimulation (EFS) were used. The muscle strips were allowed to equilibrate for 60 min. Fresh Krebs solution was added at the end of the equilibration period. After 5 minutes, a 1 mL aliquot of the bathing solution was taken and the solution was changed. Subsequently, the muscle strip was stimulated for 5 min by an electrical field known to activate the intrinsic nerves of the esophagus. Trains of stimuli were delivered at 30 sec intervals for the 5 min period. After the last stimulus, the Krebs solution was sampled (1 mL) and exchanged with new Krebs solution with experimental drugs. After a 6-h incubation, EFS was delivered at the parameters described above. The solution was sampled from each bath and exchanged with Krebs solution containing drugs. After another 5 min interval, the solution was sampled (1 mL) and EFS was undertaken. At the end of the 5 min stimulation period, a 1 mL sample of solution was taken. The tissue was removed from the bath, blotted dry, and weighed.
NO, in the presence of oxygen, is rapidly oxidized to inorganic nitrite and nitrate. In this study, the production of NO was evaluated by measuring inorganic nitrite. Since the sensitivity of most assays for nitrite is low, the nitrite was reduced to nitric oxide and measured using a chemiluminescence assay. Samples of Krebs solution taken at the times described above were placed in a chamber containing 10% potassium iodide (KI) and 25% phosphoric acid (H3PO4). The NO generated in the reduction chamber was carried by a continuous stream of pure nitrogen gas through a cold trap cooled by dry ice and acetone to a chemiluminescence NO analyzer (DASIBI model 2108, Glendale, CA, USA). The cold trap removed water vapor from the stream of gases. NO is measured in the analyzer by detection of the chemiluminescence of the reaction of NO with ozone. The output of a photomultiplier tube within the analyzer was captured by an analog-to-digital board (Mac Lab, Milford, MA, USA) in a minicomputer (Macintosh II Ci) where it was continuously displayed and integrated to quantify the amount of nitrite in each sample. The instrument was calibrated with known quantities of sodium nitrite. The reported values are measurements of inorganic nitrite only and were expressed as rates of NO production in nmol/100 mg tissue. The percent change in those values was then calculated.
Protein extracts were prepared from tissue pieces crushed and homogenized on ice in RIPA buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS] containing 0.1 mM PMSF, 1 mM sodium orthovanadate, and 10 mg/L aprotinin. Supernatant was prepared by centrifugation at 12,000 g at 4℃ for 20 min, and protein concentrations were determined with the CoomassieR protein assay reagent kit (Pierce, Rockford, IL, USA).
For iNOS or nitrotyrosine, 80 µg of soluble protein extract was loaded on an SDS/8.0 or 12% polyacrylamide gel, respectively, and separated at 25 V overnight. After electrophoretic transfer to nitrocellulose membrane, unspecific binding was blocked by incubation in TBS-T [10 mM Tris (pH 8), 150 mM NaCl, and 0.1% Tween 20] plus 4% nonfat dry milk for 1 h at room temperature. After washing in TBS-T, the membranes were incubated overnight at 4℃ with rabbit anti-iNOS or anti-nitrotyrosine polyclonal antibody (Transduction Laboratories, Lexington, KY, USA) used at a 1: 2,500 or 1:350 dilution, respectively. After washing three times in TBS-T, the membrane was probed with an anti-rabbit immunoglobulin peroxidase-coupled antibody (1:3,000) (Bio-Rad, Hercules, CA). After washing with TBS-T, positive bands were detected using an ECL Western blotting detection system (Amersham, Arlington Heights, IL, USA). Prestained molecular weight standards (Bio-Rad, Hercules, CA, USA) were used as markers. The membranes were exposed to Kodak XR film, and the film was developed. Blots were densitometrically analyzed using gel analysis software (SigmaGel, San Rafael, CA, USA).
The modified Krebs solution used in these experiments contained 138.5 mM Na+, 4.6 mM K+, 2.5 mM Ca2+, 1.2 mM Mg2+, 125 mM Cl-, 21.9 mM HCO3-, 1.2 mM H2PO4-, 1.2 mM SO4-, and 11.5 mM glucose. It was maintained at 37℃ and bubbled continuously with 95% O2-5% CO2 to maintain a pH of 7.4 throughout the experiment. Ketamine was obtained from Aveco Co., Inc. (Fort Dodge, IA, USA). Sodium pentobarbital was obtained from the University of Iowa Pharmaceutical Service. Escherichia coli lipopolysaccharide (serotype O55:B5), Cu, Zn-SOD, and catalase were obtained from Sigma Co., Inc (St. Louis, Mo, USA).
All physiological recordings were made and analyzed with MacLab software. Data are expressed as mean ± SE, and n represents the number of animals studied. The paired t-test and one way analysis of variance (ANOVA) test, if appropriate, followed by the Student-Newman-Keuls methods were used to compare means between groups. A p value of less than 0.05 was considered to be statistically significant.
The change in resting LES tension in vitro after 6 hours was significantly increased to 133.0 ± 13.7%, compared with its pretreatment values, in the LPS-treated group (p<0.05) (Fig. 1). The percent relaxation of LES by electrical field stimulation (EFS) was significantly decreased after 6 hours in the control group (PreTx., 77.0 ± 5.0%; PostTx, 63.9 ± 5.3%, p<0.05) (Fig. 2), though there was no significant change in the treated groups (Fig. 2).
The levels of tissue-bath NO concentration without stimulation were increased in all groups after 6 hours. The percent change for the LPS-treated group (290.5 ± 75.4%) was significantly less than the LPS with SOD/CAT group (100 units/mL, 716.2 ± 142.3%) or the SOD/CAT alone group (100 units/mL, 805.2 ± 123.8%) after 6 hours (Fig. 3). The EFS-induced release of NO after 6 hours in the controls was slightly decreased compared with its pretreatment values (p = 0.083) (Fig. 4).
The anti-iNOS antibody recognized a 130 kDa protein on Western blots and the expression of iNOS was detectable whether the sample was treated with LPS (n = 8, 6/8, 75.0%) or control (n = 7; 5/7, 71.4%). The band for the iNOS protein was also detected in all samples that received LPS and SOD/CAT (100 units/mL) treatment (n = 5). No iNOS protein was detected in LES muscle immediately after harvesting the tissue (before exposure to an in vitro organ bath) (n = 3).
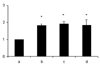
The anti-nitrotyrosine antibody recognized a 66 kDa protein, and its expression was detected in all samples from each group. The density ratio of band against that of the LES muscle immediately after harvesting the tissue (not exposed to an in vitro organ bath) was significantly increased in both the LPS and control groups (Fig. 5).
The main findings of the present study were that the resting LES tone after 6 hours was increased in the LPS-treated group, and the LES relaxation with EFS was decreased in the control group while the treated groups remained unchanged. The NO concentration in the tissue bath of LPS-treated muscle not undergoing nerve stimulation was significantly less than the LPS with SOD/CAT group or the SOD/CAT alone group. The expression of iNOS and nitrotyrosine was detectable in LES muscle of both the control and LPS-treated groups.
The superoxide anion and NO are generated during endotoxemia.3,6 Their release can be mediated and regulated by several cytokines, by bacteria-derived compounds, and endotoxins.7 We have previously shown that excessive amounts of NO are produced during endotoxemia, and this is due to upregulation of iNOS.1 One limitation of our previous experiments, however, was that administration of LPS in vivo did not allow discrimination between direct effects of LPS in the LES and systemic effects of released NO or cytokines. In contrast, the present study provides evidence that prolonged LPS exposure in vitro may be sufficient to directly induce the generation of NO and functional alterations in the LES.
Superoxide is produced in high concentrations during endotoxemia, and this production is inversely proportional to net NO production and directly related to peroxynitrite formation.3 Superoxide can react with nitric oxide, reducing the availability of this potent vasodilator and producing peroxynitrite which, while it also induces a relaxation effect, is less potent than nitric oxide, being very short-lived and highly toxic.7 Thus, the availability of nitric oxide to the smooth muscle cells and the extent of nitric oxide-dependent vasorelaxation may depend in part on the levels of superoxide. Concomitant production of NO and superoxide, a scavenger of NO, resulting from LPS exposure, make peroxynitrite, resulting in a decreased NO level.3,8 This may explain the reason why the NO level in the tissue bath solution was lower after the 6-hour exposure of LPS than in the control group.
In addition, we found increased levels of NO in the bathing solution of the control group after 6 hours. The most likely source for increased NO in the control bathing solution may be the growth of bacteria that generate nitrite. There have been reports that excessive production of NO may downregulate neuronal NOS (nNOS) expression,9 which could lead to a decrease in relaxation of the LES as well as a decreased release of NO into the tissue bath, as shown in our study. Therefore, we propose that the alterations seen in LES relaxation during physiologic experimentation may be in part due to the increased NO through induction of iNOS, as well as possible downregulation of nNOS, although our studies do not distinguish these possibilities.
In the present study, the expression of iNOS was increased in both the control and LPS-treated groups. In human myocardium, iNOS mRNA was detected in all samples from tissue bath studies, independent of whether samples were treated with endotoxin or not.10 It was suggested that endotoxin contamination of incubation basins and media could also have contributed to iNOS induction in control preparations. As previously mentioned, the growth of bacteria that generate nitrite may have a role for the increase in NO in the control bathing solution. Therefore, it is recommended that the bath medium be exchanged for fresh medium every hour during physiological experimentation.11 It has been demonstrated that the addition of an NO scavenger to the incubation medium attenuated the endotoxin effect in myocardium.10 These observations from functional experiments indicated that endotoxin effects were mediated by the formation of NO. In the present studies, an increased level of NO in the presence of SOD/CAT, a scavenger of superoxide, indicated that superoxide contributes to the inactivation of NO. Superoxide is reported to interfere with the activity of guanylate cyclase,12 and inhibition of guanylate cyclase increased resting LES tone without inhibiting nerve-induced LES relaxation.13 These are consistent with our present findings in which changes in resting LES tone were increased in the LPS-treated groups, which is thought to result from an increased state of superoxide level, although our studies did not measure the level of superoxide.
It has been reported that peroxynitrite, produced from NO and superoxide during inflammation, may produce alterations in esophageal motor function.14 Peroxynitrite is only fleetingly present under physiological conditions, and it interacts with tyrosine moieties of cellular proteins to produce a stable end product, nitrotyrosine, which is a specific nitration product of peroxynitrite.15,16 We demonstrated here that the accumulation of nitrotyrosine was detectable in the LES muscle of the control and LPS-treated group, which means NO and concomitant generation of superoxide, followed by peroxynitrite accumulation, were present under physiological conditions.
These studies suggest that NO production is increased in both control and LPS-treated tissue in vitro. Antioxidant enzymes may play a role in regulating the NO-mediated neuromuscular function of the LES.
Figures and Tables
Fig. 1
The percent change in LES resting tone after exposure to LPS and/or SOD/CAT. The percent change in LES tone is on the Y-axis. The change in tension after 6 hours was significantly increased to 133.0 ± 13.7% in the LPS (1 µg/mL)-treated group, compared with its pretreatment values. Group I, control; group II, LPS; group III, LPS with SOD/CAT 25 units/mL; group IV, LPS with SOD/CAT 100 units/mL, group V, SOD/CAT 100 units/mL. LPS, lipopolysaccharide; SOD, superoxide dismutase; CAT, catalase. Filled bar: pretreatment group, empty bar: posttreatment group. Values are mean ± SE. *p<0.05 vs pretreatment group.

Fig. 2
Effect of LPS and/or SOD/CAT on EFS-induced relaxation of the LES. Percent relaxation is on the Y-axis. The percent relaxation of the LES by EFS was significantly decreased after 6 hours in the control group (n = 12; PreTx., 77.0 ± 5.0%; PostTx, 63.9 ± 5.3%, p<0.05). Group I, control; group II, LPS (1 µg/mL); group III, LPS with SOD/CAT 25 units/mL; group IV, LPS with SOD/CAT 100 units/mL, group V, SOD/CAT 100 units/mL. EFS, electrical field stimulation (4 sec trains of 1 msec, 35 V square wave pulses at 3 Hz); LPS, lipopolysaccharide; LSC, LPS with SOD/CAT. Filled bar: pretreatment group, empty bar: posttreatment group. Values are mean ± SE. *p<0.05 vs. pretreatment group.

Fig. 3
The effect of LPS and/or SOD/CAT on levels of NO without nerve stimulation. The percent change in NO level in the tissue bath (against pretreatment level) without EFS is on the Y-axis. The NO levels were increased in all groups after 6 hours. The NO level in the LPS (1 µg/mL)-treated group (n = 10; 290.5 ± 75.4%) was significantly less than that of the LPS with SOD/CAT group (n = 8; 100 units/mL, 716.2 ± 142.3%) or the SOD/CAT alone group (n = 5; 100 units/mL, 805.2 ± 123.8%). Group I, control; group II, LPS; group III, LPS with SOD/CAT 25 units/mL; group IV, LPS with SOD/CAT 100 units/mL, group V, SOD/CAT 100 units/mL. EFS, electrical field stimulation (4 sec trains of 1 msec, 35 V square wave pulses at 3 Hz). LPS, lipopolysaccharide; SOD, superoxide dismutase; CAT, catalase. Values are mean ± SE. *p<0.05, compared with LPS with SOD/CAT 100 units/mL.

Fig. 4
The effect of LPS and/or SOD/CAT on nerve-stimulated levels of NO. The EFS-induced release of NO in the controls (n = 10) was decreased compared with its pretreatment values (p = 0.083). Group I, control; group II, LPS; group III, LPS with SOD/CAT 25 units/mL; group IV, LPS with SOD/CAT 100 units/mL, group V, SOD/CAT 100 units/mL. EFS, electrical field stimulation (4 sec trains of 1 msec, 35 V square wave pulses at 3 Hz). LPS, lipopolysaccharide; LSC, LPS with SOD/CAT; SOD, superoxide dismutase; CAT, catalase. Filled bar: pretreatment group, empty bar: posttreatment group. Values are mean ± SE.
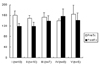
Fig. 5
Accumulation of nitrotyrosine proteins in the LES. The density ratio of band against that of LES muscle immediately after harvesting the tissue (not exposed to an in vitro organ bath) (a, n = 3) was significantly increased in the control (b, n = 7), LPS-(c, n = 8), or LPS with SOD/CAT (100 units/mL)-treated (d, n = 4) groups. *p<0.05 vs in vitro organ bath.
References
1. Park H, Cullen JJ, Clark E, Conklin JL. Effect of inhibiting iNOS on endotoxin-induced changes in esophageal motor function. Neurogastroenterol Motil. 1999. 11:278A.
2. Park H, Clark E, Cullen JJ, Conklin JL. Effect of endotoxin on opossum esophageal motor function. Neurogastroenterol Motil. 2000. 12:215–221.
3. Brovkovych V, Patton S, Brovkovych S, Kiechle F, Huk I, Malinski T. In situ measurement of nitric oxide, superoxide and peroxynitrite during endotoxemia. J Physiol Pharmacol. 1997. 48:633–644.
4. Leichus LS, Thomas RM, Murray JA, Conklin JL. Effect of oxygen radicals and radical scavenging on opossum lower esophageal sphincter. Dig Dis Sci. 1997. 42:592–596.
5. Thomas RM, Fang S, Leichus LS, Oberley LW, Christensen J, Murray JA, et al. Antioxidant enzymes in intramural nerves of the opossum esophagus. Am J Physiol. 1996. 270(1 Pt 1):G136–G142.
6. Arkovitz MS, Wispe JR, Garcia VF, Szabo C. Selective inhibition of the inducible isoform of nitric oxide synthase prevents pulmonary transvascular flux during acute endotoxemia. J Pediatr Surg. 1996. 31:1009–1015.
7. Hamilton CA, Berg G, Mcintyre M, Mcphaden AR, Reid JL, Dominiczak AF. Effects of nitric oxide and superoxide on relaxation in human artery and vein. Atherosclerosis. 1997. 133:77–86.
8. Muijsers RB, Folkerts G, Henricks PA, Sadeghi-Hashjin G, Nijkamp FP. Peroxynitrite: a two-faced metabolite of nitric oxide. Life Sci. 1997. 60:1833–1845.
9. Miller MJ, Thompson JH, Zhang XJ, Sadowska-Krowicka H, Kakkis JL, Munshi UK, et al. Role of inducible nitric oxide synthase expression and peroxynitrite formation in guinea pig ileitis. Gastroenterology. 1995. 109:1475–1483.
10. Flesch M, Kilter H, Cremers B, Laufs U, Sudkamp M, Ortmann M, et al. Effects of endotoxin on human myocardial contractility involvement of nitric oxide and peroxynitrite. J Am Coll Cardiol. 1999. 33:1062–1070.
11. Takakura K, Goto Y, Muramatsu I. Nitric oxide synthase induction and relaxation in lipopolysaccharide-treated gastric fundus muscle of rats. Life Sci. 1996. 58:9–17.
12. Brune B, Schmidt KU, Ullrich V. Activation of soluble guanylate cyclase by carbon monoxide and inhibition by superoxide anion. Eur J Bioochem. 1990. 192:683–688.
13. Murray JA, Du C, Ledlow A, Manternach PL, Conklin JL. Guanyl cyclase inhibitors: effect on tone, relaxation and cGMP content of the lower esophageal sphincter. Am J Physiol. 1992. 263:G97–G101.
14. Uc A, Murray JA, Kooy N, Conklin JL. Effect of peroxynitrite on motor functions of the opossum esophagus. Dig Dis Sci. 2001. 46:30–37.
15. Boota A, Zar H, Kim YM, Johnson B, Pitt B, Davies P. IL-1 stimulates superoxide and delayed peroxynitrite production by pulmonary vascular smooth muscle cells. Am J Physiol. 1996. 271(6 Pt 1):L932–L938.
16. Yang CC, Alvarez RB, Engel WK, Askanas V. Increase of nitric oxide synthases and nitrotyrosine in inclusion-body myositis. Neuroreport. 1996. 8:153–158.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download