Abstract
Alcohol dependence is a chronic disorder that results from a variety of genetic, psychosocial, and environmental factors. Relapse prevention for alcohol dependence has traditionally involved psychosocial and psychotherapeutic interventions. Pharmacotherapy, however, in conjunction with behavioral therapy, is generating interest as another modality to prevent relapse and enhance abstinence. Naltrexone and acamprosate are at the forefront of the currently available pharmacological options. Naltrexone is an opioid receptor antagonist and is thought to reduce the rewarding effect of alcohol. Acamprosate normalizes the dysregulation of N-methyl-D-aspartate (NMDA)-mediated glutamatergic excitation that occurs in alcohol withdrawal and early abstinence. These different mechanisms of action and different target neurotransmitter systems may endow the two drugs with efficacy for different aspects of alcohol use behavior. Since not all patients seem to benefit from naltrexone and acamprosate, there are ongoing efforts to improve the treatment outcomes by examining the advantages of combined pharmacotherapy and exploring the variables that might predict the response of the medications. In addition, novel medications are being investigated to assess their efficacy in preventing relapse and increasing abstinence.
Alcohol dependence is a chronic disorder that results from a variety of genetic, psychosocial, and environment factors.1 Over the last 20 years, there has been considerable progress in efforts to reduce the enormous alcohol related costs to society, such as traffic accidents in which the driver is intoxicated, and to set boundaries for injudicious alcohol use in Korea. These socio-cultural changes have probably been successful in lowering the prevalence of alcohol abuse but the prevalence of alcohol dependence seems to have been less affected.2 In a recent Korean epidemiological study,3 it was established that 10.20% of the adult population has a lifetime prevalence of alcohol dependence (15.97% of men and 4.64% of women) which makes alcohol dependence the second most common psychiatric disorder in Korea.
Treating alcohol dependence usually consists of two phases: detoxification and rehabilitation. The initial detoxification stage deals with acute withdrawal symptoms. The later rehabilitation stage attempts to prevent relapse and develops a lifestyle compatible with long-term abstinence. Whereas detoxification is widely accepted as a pharmacotherapeutic domain, rehabilitation, in clinical practice, has traditionally involved psychosocial and psychotherapeutic interventions consisting of individual and group psychotherapy, cognitive-behavioral treatments, and self-directed groups such as Alcoholics Anonymous. Although psychosocial treatments have shown effectiveness in reducing alcohol consumption and maintaining abstinence, 40 to 70% of patients still relapse to drinking within a year following treatment.4 As a part of the efforts to improve the treatment outcomes for alcohol dependence, pharmacotherapy is being investigated as another modality to enhance abstinence and prevent relapse, complementing psychosocial interventions.
The rationale of using pharmacotherapy for alcohol dependence is based on several premises.5,6 First, advances in neurobiology have identified the neurobehavioral effects of alcohol and their associated neurotransmitter systems which are related to the development of dependence and, at the same time, are potential targets for pharmacological approaches. Second, recent genetic studies have confirmed that alcohol dependence is a heterogeneous condition. While some gene variations predispose people to alcohol dependence, others confer protection. Third, animal models of alcohol dependence relapse have demonstrated that pharmacologic agents can reduce alcohol consumption and have proven to be fairly predictive of similar responses in human patients. Fourth, medications have improved the treatment of other addictive disorders such as bupropion for nicotine dependence and methadone for heroin dependence, encouraging pharmacotherapies for the treatment of alcohol dependence.
To date, three medications - disulfiram, naltrexone, and acamprosate - have been approved by the U.S. Food and Drug Administration (FDA) for treatment of alcohol dependence (Table 1). Only about 20% of eligible patients receive them, however.7 Unfortunately, medications are still disparaged as a "crutch" and some still stick to the old adage, "You can't treat a drug problem with a drug."8
This article discusses (1) the neurobiological basis of alcohol dependence; (2) the efficacy and safety of disulfiram, naltrexone and acamprosate, the approved pharmacotherapies for alcohol dependence; (3) the ongoing issues for improving the effectiveness of pharmacotherapy, and (4) the novel pharmacotherapies currently under investigation.
For many years, it has been suggested that alcohol exerts its neurobiological effects mainly by increasing membrane fluidity, altering the function of macro-molecules in the cell membrane. New evidence, however, indicates that alcohol binds to hydrophobic pockets of proteins, modulating their function by changing their 3-dimensional structure. Proteins that are particularly sensitive to this effect include ion-channels, neurotransmitter receptors, and enzymes involved in signal transduction.9 Neurotransmitters with notable sensitivity to this effect include dopamine, serotonin, gamma-aminobutyric-acid (GABA), glutamic acid, adenosine, neuropeptide Y, norepinephrine, cannabinoid receptors, and opioid peptides.10 These neurotransmitter systems are involved in the different components of alcohol dependence and are therefore targets for pharmacotherapeutic interventions.
Considerable evidence has emerged suggesting that the dopamine system plays a central role in the biology of alcoholism. Mesolimbic dopamine A10 neurons are activated by alcohol, resulting in a release of the neurotransmitter in the nucleus accumbens and mediating positive reinforcement and reward.11 It is postulated that repeated alcohol use sensitizes the system, so that behavioral stimuli associated with alcohol also cause the release of dopamine and facilitate additional alcohol use.12 This sensitization may account for the craving and preoccupation with alcohol that are the hallmarks of alcohol dependence.
The endogenous opioid system seems to play a modulatory role on the dopaminergic system, whereby activation of opiate receptors stimulates the release of dopamine in the brain. Alcohol consumption increases the release of endorphins (which are endogenous opioid peptides) in the brain, thus indirectly activating the dopaminergic reinforcement/reward system.13 It has been postulated that individual differences in the sensitivity of endogenous opioid systems may underlie individual differences in the intensity of alcohol craving and the risk of becoming alcohol dependent.
The facilitation of inhibitory GABAergic and the inhibition of excitatory glutamatergic neurotransmission are important targets for the acute effects of alcohol. Potentiation of GABAergic inhibition is widely accepted as the underlying cause of the acute sedative effects of alcohol. Long-term adaptive changes to the sedative effects of alcohol in these two neurotransmitter systems are thought to underlie the development of alcohol dependence. After chronic exposure to alcohol, there is a compensatory up-regulation of the glutamatergic system (and down-regulation of the GABA system) in an attempt to balance alcohol's inhibitory action. The result is an increased tolerance for alcohol.14 When alcohol is abruptly withdrawn, however, a state of hyper-excitability emerges. This is perceived by the patient as a disagreeable state of arousal, anxiety and sleeplessness and is the core of the negative affective state which the alcoholic patient will drink to relieve. These plastic changes in the brain, brought about by change in protein synthesis, are only slowly reversible. This may explain the persistence of negative craving during alcohol withdrawal and why stable abstinence after acute detoxification is so difficult to achieve. Antiglutamamatergic agents, such as NMDA antagonists and anticonvulsant agents, have been proposed to reduce the motivation for drinking by suppressing symptoms of alcohol withdrawal. Recent data suggest that NMDA antagonists may have other beneficial effects in alcohol dependent patients, such as substituting for deficits in negative feedback signals or reducing the development of tolerance/sensitization to alcohol.15,16
The first agent to be approved for treatment of alcohol dependence was disulfiram. This substance was serendipitously discovered to be an agent causing alcohol aversion in Ohio rubber workers in 1939. The major metabolic pathway for alcohol metabolism is a two-step enzymatic process (ethanol → acetaldehyde → acetic acid). Disulfiram is an irreversible inhibitor that blocks the second stage of alcohol metabolism, causing an accumulation of toxic intermediate acetaldehyde, which results in hypotension, flushing, nausea, and vomiting. The objective of disulfiram treatment is thus to create an aversion to alcohol, rather than to modulate its neurochemical effects. Although many studies have been performed with disufiram, controlled clinical trials demonstrated inconsistent findings for alcohol drinking outcomes between disulfiram and placebo, and have failed to clearly establish the therapeutic benefit of this treatment in enhancing abstinence.17 However, it is difficult to conclude the efficacy of the treatment through classical double-blind, placebo-controlled trials, since it is the psychological deterrent effect of the drug rather than its biological effect that is useful.18 Fuller et al.19 observed no significant differences in abstinence rates or in the time to first relapse among groups taking placebo, 1 mg/day disulfiram (an inactive dose) or 250 mg/day disulfiram (the standard dose). The patients receiving 250 mg disulfiram, however, had fewer drinking days once they relapsed than did the other two groups.
The disulfiram dosage is usually 250 mg per day with a maximum of 500 mg per day. While normal results of alcohol consumption after taking disulfiram are palpitations, flushing, nausea, vomiting and headaches, more severe reactions could include myocardial infarction, congestive heart failure, respiratory depression, and death. The use of disulfiram appears to be most useful in adherent patients, in special high-risk patients, and when administration is supervised.
Naltrexone and acamprosate are at the forefront of currently available pharmacological options and they share many important features20 (Table 2). Most of the concerns in using medications for relapse prevention are probably related to the possible intrinsic dependence potential. However, there is no evidence that naltrexone or acamprosate have developed either tolerance or withdrawal symptoms, including rebound drinking when treatment is ceased, and nor do they have any overt psychoactive effects on the central nervous system. In addition, both drugs do not have pharmacological or pharmacokinetic interaction with alcohol and so the serious side effects associated with disulfiram are not troublesome when using naltrexone or acamprosate.
The important difference between naltrexone and acamprosate are mainly attributed to their mechanisms of action. Naltrexone is an antagonist at the opioid receptors, which are known to mediate the rewarding effects of alcohol, and is thus thought to reduce the desire or craving for rewarding. Although less well defined, acamprosate normalizes the dysregulation of NMDA-mediated glutamatergic excitation that occurs in alcohol withdrawal and early abstinence. This effect probably attenuates the desire or craving for reduce of tension. These different mechanisms of actions may endow the two drugs with efficacy for different components of drinking.
The USA FDA's approval of naltrexone for the treatment of alcohol dependence was based mainly on two small, double-blind, placebo-controlled trials demonstrating a reduced rate of relapse to heavy drinking, reduced craving, and less frequent drinking in naltrexone-treated patients.22,23 During the following years, several more trials have followed and three meta-analyses have concluded that naltrexone is efficacious in the treatment of alcohol dependence.24-26 The most consistent finding obtained with naltrexone is an increased time to first relapse (typically defined as more than 5 drinks/day in males, 4 drinks/day in females). The decrease in relapse rate, however, has not been observed in all studies. Such differences in results are seen even in the largest trial.27 Several factors may explain the discrepancies in results of the different clinical trials of naltrexone. The animal models of relapse indicate that naltrexone blocks cue-induced relapse but is less effective against stress-induced relapse.28 The effect of naltrexone on relapse may, to some extent, be dependent on associated psychotherapy, since naltrexone was found to be more effective in patients receiving training in coping skills than in those receiving supportive therapy alone.29 Compliance may be a limiting factor in naltrexone treatment.30-32 Monti et al.32 demonstrated a significant treatment outcome only when non-compliant subjects were excluded from the analysis. A large multi-site, double blind, placebo-controlled trial with an injectable, sustained-release formation, (a strategy to improve compliance) demonstrated that relapse to heavy drinking decreased in patients receiving depot preparation compared to placebo.33
A conceptual framework for integrating the clinical data on naltrexone has been proposed by Sinclair,34 who suggested that naltrexone is useful for preventing relapse rather than at maintaining absolute abstinence. Thus, the contingency of drinking alcohol and taking naltrexone is important in bringing to light treatment effects. In a recent systematic meta-analysis of 24 placebo-controlled trials, including a total of 2861 patients, short-term naltrexone therapy significantly decreased relapse rate (relative risk 0.64), but did not enhance absolute abstinence (relative risk 0.91).35
For the first 90 days of abstinence, when the risk of relapse is greatest, the recommended dosage of naltrexone is a single dose of 50 mg per day but doses of 25 mg to 100 mg daily are sometimes used. The most common side effects are nausea (10%), headache (7%), anxiety (2%) and sedation (2%).36 Naltrexone has been shown to have dose-related hepatotoxicity, although generally this occurs at doses of 300 mg per day, higher than those recommended for treatment of alcohol dependence. The drug is contraindicated in patients with hepatitis or liver failure, and a monthly check of hepatic transaminase levels is recommended, for the first three months and every three months thereafter.
Acamprosate was investigated in nearly 20 controlled, published trials with about 4000 patients and these studies have produced consistent results showing that acamprosate treatment is superior to placebos in maintaining abstinence.37 In all but three clinically controlled published studies, the proportion of acamprosate-treated patients abstaining at the end of the study was twice as high as patients receiving placebos. In addition, two studies38,39 evaluated long-term abstinence for 1-ear after the end of the treatment period, and both showed that treatment effects were maintained. A systematic metaanalysis37 in which clinical data from 17 trials were reanalyzed concluded that the treatment effect could increase with time.
Since most of the trials were undertaken in Europe, it was considered important to evaluate the efficacy and safety of acamprosate in different ethnic groups, given the emerging role of genetic and cultural issues in drug treatment. In a study by Namkoong et al.,40 however, acamprosate did not show any treatment benefits when compared to the placebo. This negative finding might be explained by the sample characteristics (i.e., a more severe form of alcohol dependence, a lower level of social support, a short interval between the last drink and the first medication), the dosage issues of acamprosate, the short study period (8 weeks), and the variable concomitant psychosocial treatment.
Acamprosate is available in 333-mg enteric coated tablets. Dosing is determined by weight (≥ 60 kg: 1998 mg, < 60 kg: 1332 mg). It is not metabolized but is eliminated by renal excretion, and should therefore be given cautiously with renal impairment. Acamprosate is well tolerated with limited side effects, most commonly transient diarrhea (10%) and headache (20%). Like naltrexone and disulfiram, acamprosate is FDA pregnancy category C, i.e., there have been adverse effects on the fetus in animal studies but no human trials have been performed.
Combining naltrexone and acamprosate in the treatment of alcohol dependence is an attractive concept for several reasons. Since naltrexone and acamprosate have different mechanisms of action and different target neurotransmitter systems, presumably, they affect different aspects of alcohol use behavior. (Naltrexone decreases alcohol consumption and acamprosate stabilizes abstinence.) Pharmacokinetic and behavioral assessments of combining naltrexone and acamprosate have found the combination to be safe.
Kiefer et al.41 performed a randomized, double-blind, placebo-controlled, clinical trial of 160 alcohol-dependent patients and assessed the efficacy of naltrexone and acamprosate, as monotherapy and in combination. It was demonstrated that the proportion of patients remaining absolutely abstinent at the end of the 12-week treatment period was around twice as high in the combination therapy group than in the monotherapy group (placebo 25%, naltrexone alone 65%, acamprosate alone 50%, combination therapy 73%). Even though further relapse occurred during the follow-up period, the relative treatment benefits between the three treatment groups and the placebo group was maintained at the end of the 3-month open label phase. There was, however, no significant difference between the three treatment groups (placebo 20%, naltrexone alone 47%, acamprosate alone 46%, combination therapy 66%).42 Although combination therapy was generally well tolerated, the incidence of diarrhea (13.8%) and nausea (5.6%) was significantly greater than in the monotherapy groups, perhaps due to a pharmacokinetic interaction.
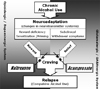
There are several possible explanations for the superior efficacy of the combination treatment.43 First, there may be subgroups that respond selectively to naltrexone or acamprosate and thus the added benefit of combination therapy would be merely explained by the recruitment of additional responder patients. A hypothesis of such patient subgroups may be that 'reward' craving drinkers would respond better to naltrexone and 'relief' craving drinkers would respond better to acamprosate (Fig. 1). Attempts to find variables that might predict the response of medications will be discussed later. Second, the combination produces a synergic anticraving effect as the two drugs interfere with distinct biological aspects of the craving process. Third, pharmacokinetic interaction might underlie the observed treatment benefits, whereby bioavailability might be enhanced by co-administration of the other drug.
The ongoing COMBINE study44 plans to recruit 1,375 subjects at 11 sites to examine treatment interactions between naltrexone, acamprosate and two behavioral interventions (medical management and combined behavioral interventions). The data from COMBINE will answer important questions regarding the effectiveness of naltrexone and acamprosate both alone and in combination, as well as that of psychosocial treatment.
Since not all patients seem to benefit from the different anticraving drugs, the use of matching procedures might be important in improving the effectiveness of treatment with anticraving compounds.45 The type of craving could possibly be an important predictor or matching variable with anticraving compounds. In general, craving mainly refers to the strong desire or urge to experience the effect of a previously experienced psychoactive substance.46 Despite the simplicity of this definition, a wide variety of craving concepts are used in the research and clinical field. Verheul et al.47 have proposed a novel three pathway model of craving in alcoholics. This model suggests that craving is likely to result from distinct psychobiological mechanisms and that the efficacy of different anti-craving compounds is associated with individual differences in craving: (1) The reward pathway suggests that craving or desire for the rewarding, stimulating and/or enhancing effects of alcohol might result from either dopaminergic/opioidergic dysregulation or a personality style characterized by reward seeking and/or hedonism. (2) The relief pathway suggests that craving or desire for the reduction of tension, arousal or withdrawal might result from either GABAergic/glutamatergic dysregulation or a personality style characterized by stress reactivity, anxiety sensitivity, and/or hyperarousability. (3) The obsessive pathway can be defined as a lack of control over intrusive thoughts about drinking resulting in impaired functioning. This pathway of craving might result from serotonin deficiency or a personality style characterized by low constraint or disinhibition.
Some studies found that patients with high levels of alcohol craving are most likely to benefit from naltrexone treatment.48,49 No distinction, however, was made between reward, relief and obsessive craving. Palfai et al.50 conducted a study among hazardous drinkers and suggested that naltrexone may be particularly effective in reducing the cue-elicited positive reinforcement of alcohol for those with high positive alcohol outcome expectancies. Positive outcome expectancies also moderated the effects of naltrexone on subjective reports of stimulation following drinking.
Lesch et al.51 conducted a long-term, prospective study to assess the efficacy of acamprosate based on the four subtypes of alcohol dependent-patients. Consistent with their matching hypothesis, based on the three pathway model of Verheul et al.,48 acamprosate differentially reduced alcohol intake in those patients who use alcohol to counteract withdrawal symptoms (Type I), and in patients who use alcohol as a conflict-solving and anxiety reducing agent (Type II), but not in patients who ingest alcohol to self-medicate affective disorders (Type III) or patients with a history of cerebral impairment that precedes the development of alcohol dependence (Type IV). A recent study including the pooled data of 1,485 alcohol-dependent patients from seven randomized, controlled trials comparing acamprosate and a placebo, directly tested the matching hypothesis that acamprosate would be more effective in patients with high physical dependence at baseline, negative family history of alcoholism, late age of onset, serious anxiety symptomatology at baseline, severe craving at baseline, and female gender.52 In contrast to the expectations, the authors found that none of these theoretically relevant clinical matching variables predicted the treatment effectiveness of acamprosate. Similarly, another pooled analysis of 11 trials by Sass et al.53 did not find any demographical, psychopathological or biological predictors for acamprosate efficacy.
It should be noted that the three-pathway model by Verheul et al.48 and related matching hypotheses might not cover all the possible mechanisms of action of anti-craving medications. For example, a recent study showed that naltrexone not only includes an opioid-receptor blockade, but that it indirectly increased hypothalamic pituitary adrenocortical (HPA) activity, resulting in higher adrenocorticotropic hormone (ACTH) and cortisol levels, which may in turn be partially responsible for the effect of naltrexone on drinking and craving.54
Investigating the role of genetics in predicting treatment outcomes is one promising area of research since a genetic basis of for alcoholism (heritability rate, 50-60%) is well established.55 Monterossso et al.49 explored the predictors of naltrexone response in a double-blind, placebo-controlled trial and demonstrated significant interactions between treatment condition and family history of alcohol problems. Some studies have investigated individual differences with respect to sensitivity in the hypothalamic pituitary adrenal axis to naltrexone administration. King et al.56 examined the neuroendocrine and mood responses to oral naltrexone as a function of the biological family history of alcoholism. The results demonstrated the potential biological bases of altered opioidergic sensitivity in those persons with an assumed greater inherited vulnerability for future alcoholism. Meanwhile, studies of family history as a predictor of treatment response have led to speculation that naltrexone may function differently in genetically predisposed individuals.
Naltrexone has a high affinity for the µ-opioid receptor, which is hypothesized to be the principal site of action of the drug. It has been hypothesized that sequence variation in the gene encoding the µ-receptor may result in a receptor with altered expression, structure, or function, and as a consequence, may increase or decrease an individual's susceptibility to substance dependence.57 Oslin et al.58 examined the association between two specific polymorphisms of the gene encoding the µ-opioid receptor and treatment outcomes, measured over 12 weeks, in alcohol dependent patients who were prescribed naltrexone or a placebo. Patients with one or two copies of the Asp40 allele treated with naltrexone had significantly lower rates of relapse (26.1% versus 47.9%; p = 0.0044) and took a longer time to return to heavy drinking than those homozygous for the Asn40 allele. There were no differences in relapse rates or abstinence rates between the two genotype groups among those assigned to placebo. Meanwhile, Kim et al.59 reported that the allele frequency of the Asp40 allele was 39.7% in the Korean alcohol dependent group, which is consistent with previous studies demonstrating a higher Asp40 allele frequency in the Asian population. Within the alcohol dependent group, the Asp40 allele was associated with more drinking days. The finding of a genotype that predicts success with naltrexone, if replicated, will provide a pharmacogenetic tool to enhance the matching of patients to treatment and encourage the search for additional functional polymorphisms.
During the last two decades, a number of drugs acting on serotonergic neurotransmission have been studied in alcohol dependence, since serotonin is widely implicated in a variety of consummatory behaviors and impulsivity. These agents are either selective serotonin reuptake inhibitors or receptor agonist/antagonists. Most of this work, however, has used small samples with relatively short treatment periods. Selective serotonin reuptake inhibitors, despite their effectiveness in animals, have shown inconsistent or disappointing results in humans60-65 and so the usefulness is still controversial.17 Meanwhile, no evidence of clinical efficacy in alcohol-dependent has been obtained with ritanserin (5-HT2 anatagonist).66,67 In addition, a meta-analysis of studies performed with bupropion (5-HT1 partial agonist) concluded that any efficacy of bupropion was secondary to an anxiolytic effect, rather than on drinking perse.68
Of the numerous serotonergic drugs which have been suggested as pharmacotherapies for alcohol dependence treatment, ondansetron, a 5-HT3 antagonist that is FDA-approved as an antiemetic, appears to be the most promising.69 The 5-HT3 receptor is involved in the expression of alcohol's rewarding effects. Behavioral pharmacological studies show that many of the reward effects of alcohol are mediated by interactions between DA and the 5-HT3 receptor in the mid-brain and cortex.70,71 5-HT3 receptors are densely distributed in the terminals of mesocorticolimbic DA-containing neurons where they regulate DA release in these brain regions. Following a previous clinical trial,72 Johnson et al.73 evaluated ondansetron as a treatment for alcohol dependence in a 12-week, double-blind, placebo-controlled trial of 321 patients. The early-onset, alcohol dependent group treated with ondansetron (particularly 4 µg/kg b.i.d.) reported fewer drinks per day and fewer drinks per drinking days, while the late-onset group treated with ondansetron did not differ from those treated with placebo. It is interesting that while serotonine reuptake inhibitors have little effect on drinking among early-onset alcoholics, ondansetron, with functionally opposite effects in the serotonergic system, is effective for the early-onset subtype. Sufficient evidence exists that early-onset alcoholics are more prone to serotonergic dysfunction than late-onset alcoholics.74,75
Mood stabilizers and anticonvulsants decrease alcohol consumption in experimental animals. Clinical trials, however, have not provided clear evidence of the efficacy of treatment for alcohol dependence. The controlled trials of lithium did not demonstrate efficacy in either non-depressed or depressed alcohol-dependent patients and Garbutt et al.17 concluded that lithium lacks efficacy in the treatment of primary alcohol dependence. More promising are results with non-benzodiazepine anticonvulsants such as carbamazepinem, valproate, gabapentin, vigabatrin and topiramate.76
Topiramate, although only approved by the FDA for seizure disorders, was evaluated in the treatment of alcohol dependence because of its effects on GABAergic and glutamatergic systems. It has been shown to augment GABA function and inhibit specific glutamatergic pathways at the AMPA/kinate receptors. This may decrease the cortical expression of alcohol reward by decreasing the midbrain dopamine function.77 In a 12-week double-blind, placebo-controlled trial of 150 alcohol dependent patients, topiramate was more effective than placebo in initiating abstinence and in reducing self-reported drinks per day, drinks per drinking days, and heavy drinking days. Compared with a placebo, it also significantly reduced craving as assessed with Obsessive Compulsive Drinking Scale. Topiramate was equally effective in both early-onset and late-onset alcohol dependence.78 The study used an escalating dose of 25 to 300 mg per day. Hypersensitivity to the drug was the only known contraindication. No serious adverse events occurred. Paresthesia was the most common adverse effect, which includes dizziness, somnolence, diplopia and nausea.
Important advances have been made in the development of pharmacotherapy in alcohol dependence and it is experiencing a major shift in direction. There is clear evidence of the efficacy and safety of natrexone and acamprosate, and still more novel medications are under investigation. In addition, important questions remain regarding the optimal dose and duration of treatment, the role of combinations of medications, and the treatment subtypes of alcohol dependent patients.
Figures and Tables
Fig. 1
A representation of the neuroadaptive model of craving and possible mechanisms of naltrexone and acamprosate.
References
1. Morse RM, Flavin DK. The definition of alcoholism. The Joint Committee of the National Council on Alcoholism and Drug Dependency and the Amercian Society of Addiction Medicine to Study the Definition and Criteria for the Diagnosis of Alcoholism. JAMA. 1992. 268:1012–1014.
2. Jung YC, Namkoong K. Two alcoholisms: Abuse and Dependence: Nosology issues from the epidemiological studies of alcoholism in Korea. Psychiatr Invest. 2005. 2:40–52.
3. Cho MJ, Hahm BJ, Kim JK, Park KK, Chung EK, Suh TW, et al. Korean Epidemiologic Catchment Area (KECA) Study for Psychiatric Disorders: Prevalence of Specific Psychiatric Disorders. J Korean Neuropsychiatr Assoc. 2004. 43:470–480.
4. Finney JW, Hahn AC, Moos RH. The effectiveness of inpatient and outpatient treatment for alcohol abuse: the need to focus on mediators and moderators of setting effects. Addiction. 1996. 91:1773–1796.
5. Swift RM. Drug therapy in alcohol dependence. N Engl J Med. 1999. 340:1482–1490.
6. Kenna GA, McGeary JE, Swift RM. Pharmacotherapy, pharmacogenomics, and the future of alcohol dependence treatment, Part 2. Am J Health Syst Pharm. 2004. 61:2272–2388.
7. Williams SH. Medications for treating alcohol dependence. Am Fam Physician. 2005. 72:1775–1780.
8. O'Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005. 162:1423–1431.
9. Gordis E. The neurobiology of alcohol abuse and alcoholism: building knowledge, creating hope. Drug Alcohol Depend. 1998. 51:9–11.
10. Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, et al. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998. 22:3–9.
11. Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985. 348:201–203.
12. Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993. 18:247–291.
13. Benjamin D, Grant E, Pohorecky LA. Naltrexone reverses ethanol-induced dopamine release in the nucleus accumbens in awake, freely moving rats. Brain Res. 1993. 621:137–140.
14. Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol. Psychopharmacology. 1998. 139:2–19.
15. Quertemont E, Brabant C, De Witte P. Acamprosate reduces context-dependent ethanol effects. Psychopharmacology. 2002. 164:10–18.
16. Kiefer F, Jahn H, Tarnaske T, Helwig H, Briken P, Holzbach R, et al. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2003. 60:92–99.
17. Garbutt JC, West Sl, Carey TS, Lohr KN, Crews FT. Pharmacological treatment of alcohol dependence: a review of the evidence. JAMA. 1999. 281:1318–1325.
18. Kiefer F, Mann K. New achievements and pharmacotherapeutic approaches in the treatment of alcohol dependence. Eur J Pharmacol. 2005. 526:163–171.
19. Fuller RK, Brachey l, Brightwell DR, Derman RM, Emrick CD, Iber FL, et al. Disulfiram treatment of alcoholism. A Veterans Administration Cooperative Study. JAMA. 1996. 256:1449–1455.
20. Mason BJ. Acamprosate and naltrexone treatment for alcohol dependence: an evidence-based risk-benefits assessment. Eur Neuropsychopharmacol. 2003. 13:469–475.
21. Saivin S, Hulot T, Chabac S, Potgieter A, Durbin P, Houin G, et al. Clinical pharmacokinetics of acamprosate. Clin Pharmacokinet. 1998. 35:331–345.
22. Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992. 49:876–880.
23. O'Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry. 1992. 49:881–887.
24. Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcohol Clin Exp Res. 2001. 25:1335–1341.
25. Streeton C, Whelan G. Naltrexone, a relapse prevention maintenance treatment of alcohol dependence: a meta-analysis of randomized controlled trials. Alcohol Alcohol. 2001. 36:544–552.
26. Srisurapanont M, Jarusuraisin N. Opioid antagonist for alcohol dependence. Cochrane Database Syst Rev. 2002. (2):CD001867.
27. Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Veterans Affairs Naltrexone Cooperative Study 425 Group. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001. 345:1734–1739.
28. Liu X, Weiss F. Addictive effect of stress and grug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanism. J Neurosci. 2002. 22:7856–7861.
29. Balladin J, Berglund M, Borg S, Mansson M, Bendtsen P, Franck J, et al. A 6-month controlled naltrexone study: combined effect with cognitive behavioral therapy in outpatient treatment of alcohol dependence. Alcohol Clin Exp Res. 2003. 27:1142–1149.
30. Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy of outpatients alcoholics: results of a placebo-controlled trial. Am J Psychiatry. 1999. 156:1758–1764.
31. Chick J, Anton R, Checinski K, Croop R, Drummond DC, Farmer R, et al. A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol Alcohol. 2000. 35:587–593.
32. Monti PM, Rohsenow DJ, Swift RM, Gulliver SB, Colby SM, Mueller TI, et al. Naltrexone and cue exposure with coping and communication skills training for alcoholics: treatment process and 1-year outcomes. Alcohol Clin Exp Res. 2001. 25:1634–1647.
33. Garbutt JC, Kranzler HR, O'Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005. 293:1617–1625.
34. Sinclair JD. Evidence about the use of naltrexone for different ways of using it in the treatment of alcoholism. Alcohol Alcohol. 2001. 36:2–10.
35. Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005. 8:267–280.
36. Croop RS. The Naltrexone Usage Study Group. The safety profile of naltrexone in the treatment of alcoholism Results from a multicenter usage study. Arch Gen Psychiatry. 1997. 54:1130–1135.
37. Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in maintaining abstinence in alcohol dependent individuals: results of meta-analysis. Alcohol Clin Exp Res. 2004. 28:51–63.
38. Sass H, Soyka M, Mann K, Zieglgansberger W. Relapse prevention by acamprosate. Results from a placebo controlled study in alcohol dependence. Arch Gen Psychiatry. 1996. 53:673–680.
39. Whitworth AB, Fischer F, Lesch OM, Nimmerrichter A, Oberbauer H, Platz T, et al. Comparison of acamprosate and placebo in long-term treatment of alcohol dependence. Lancet. 1996. 347:1438–1442.
40. Namkoong K, Lee BO, Lee PG, Choi MJ, Lee E. Acamprosate in Korean alcohol-dependent patients: a multi-centre, randomized, double blind, placebo controlled study. Alcohol Alcohol. 2003. 38:135–141.
41. Keifer F, Jahn H, Tarnaske T, Helwig H, Briken P, Holzbach R, et al. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2003. 60:92–99.
42. Kiefer F, Andersohn F, Otte C, Wolf K, Jahn H, Wiedemann K. Long-term effects of pharmacotherapy on relapse prevention in alcohol dependence. Acta Neuropsychiatr. 2004. 16:233–238.
43. Kiefer F, Wiedemann K. Combined therapy: what does acamprosate and naltrexone combination tell us? Alcohol Alcohol. 2004. 39:542–547.
44. COMBINE study group. Testing combined pharmacotherapies and behavioral interventions in alcohol dependence (the COMBINE study): rationale and methods. Alcohol Clin Exp Res. 2003. 27:1107–1122.
45. Ooteman W, Verheul R, Naassila M, Daoust M, Schippers GM, Koeter MWJ, et al. Patient-treatment matching with anti-craving medications in alcohol dependent patients: A review on phenotypic, endophenotypic and genetic indicators. J Substance Use. 2005. 10:75–96.
46. Anton RF. What is craving? Models and implications for treatment. Alcohol Res Health. 1999. 23:165–173.
47. Verheul R, van den Brink W, Geerlings P. A three-pathway psychobiological model of craving for alcohol. Alcohol Alcohol. 1999. 34:197–222.
48. Volpicelli JR. Naltrexone in the alcohol dependence. Lancet. 1995. 346:456.
49. Monterosso JR, Flannery BA, Pettinati HM, Oslin DW, Rukstalis M, O'Brein CP, et al. Predicting treatment response to naltrexone: The influence of craving and family history. Am J Addiction. 2001. 10:258–268.
50. Palfai T, Davidson D, Swift R. Influence of naltrexone on cue-elicited craving among hazardous drinkers: the moderational role of positive outcome experiences. Exp Clin Psychopharmacol. 1999. 7:266–273.
51. Lesch OM, Walter H. Subtypes of alcoholism and their role in therapy. Alcohol Alcohol. 1996. 31:63–67.
52. Verheul R, Lehert P, Geerlings PJ, Koeter MW, Van den Brink W. Predictors of acamprosate efficacy: results from a pooled analysis of seven European trials including 1485 alcohol-dependent patients. Psychopharmacology. 2005. 178:167–173.
53. Sass H, Soyka M, Mann K, Zieglgansberger W. Relapse prevention by acamprosate. Results from a placebo-controlled study on alcohol dependence. Arch Gen Psychiatry. 1996. 53:673–680.
54. O'Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamic-pituitary-adrenocortical axis. Psychopharmacology(Berl). 2002. 160:19–29.
55. Heath AC. Genetic influences on alcoholism risk: a review on adoption and twin studies. Alcohol Health Res World. 1995. 19:166–171.
56. King AC, Schluger J, Gunduz M, Borg L, Perret G, Ho A, et al. Hypothalamic-pituitary-adrenocortical (HPA) axis response and biotranformation of oral naltrexone: preliminary examination of relationship to family history of alcoholism. Neuropsychopharmacology. 2002. 26:778–788.
57. Lichtermann D, Franke P, Maier W, Rao ML. Pharmacogenetics and addiction to opiates. Eur J Pharmacol. 2000. 410:269–279.
58. Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Vopicelli JR, et al. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003. 28:1546–1552.
59. Kim SG, Kim CM, Kang DH, Kim YJ, Byun WT, Kim SY, et al. Association of functional opioid receptor genotypes with alcohol dependence in Koreans. Alcohol Clin Exp Res. 2004. 28:986–990.
60. Gorelick DA, Paredes A. Effects of fluoxetine on alcohol consumption in male alcoholics. Alcohol Clin Exp Res. 1992. 16:261–265.
61. Kabel DI, Petty F. A placebo-controlled, double-blind study of fluozetine in severe alcohol dependenc: adjunctive pharmacotherapy during and after inpatient treatment. Alcohol Clin Exp Res. 1996. 20:780–784.
62. Kranzler HR, Burleson JA, Korner P, Del Boca FK, Bohn MJ, Brown J, et al. Placebo-controlled trial of fluoxetine as an adjunct to relapse prevention in alcoholics. Am J Psychiatry. 1995. 152:391–397.
63. Janiri L, Gobbi G, Mannelli P, Pozzi G, Serretti A, Tempesta E. Effects of fluoxetine at antidereesant doses on short-term outcome of detoxified alcoholics. Int Clin Psychopharmacol. 1996. 11:109–117.
64. Pettinati HM, Volpocelli JR, Luck G, Kranzler HR, Rukstalis MR, Cnaan A. Double-blind clinical trial of sertraline treatment for alcohol dependence. J Clin Psychopharmacol. 2001. 21:143–153.
65. Roy A. Placebo-controlled study of setaline in depressed recently abstinent alcoholics. Biol Psychiatry. 1998. 44:633–637.
66. Johnson BA, Jansinski DR, Galloway GP, Kranzler H, Weinreib R, Anton RF, et al. Ritanserin in the treatment of alcohol dependence-a multi-center clinical trial. Psychopharmacology. 1996. 128:206–215.
67. Wiesbeck GA, Weijers JG, Chick J, Naranjo CA, Boening J. Ritanserin in Alcoholism Work Group. Ritanserin in relapse prevention in abstinent alcoholics: results from a placebo-controlled, double-blind internaltional multi-center trial. Alcohol Clin Exp Res. 1999. 23:230–235.
68. Malec TS, Malec EA, Dongier M. Efficacy of bupropion in alcohol dependence: a review. Alcohol Clin Exp Res. 1996. 20:853–858.
69. LeMarquand D, Pihl RO, BenKelfat C. Serotonin and alcohol intake, abuse, and dependence: clinical evidence. Biol Psychiatry. 1994. 36:326–337.
70. Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999. 38:1083–1152.
71. Johnson BA, Cowen PJ. Alcohol induced reinforcement: dopamine and 5-HT3 receptor interactions in animals and humans. Drug Dev Res. 1993. 30:153–169.
72. Sellers EM, Toneatto T, Romach MK, Somer GR, Sobell LC, Sobell MB, et al. Clinical efficacy of the 5-HT3 antagonist ondansetron in alcohol abuse and dependence. Alcohol Clin Exp Res. 1995. 18:879–885.
73. Johnson BA, Roache JD, Javors MA, Diclemente CC, Cloninger CR, Prihoda TJ, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000. 284:963–971.
74. Fils-Aime ML, Eckardt MJ, George DT, Brown GL, Mefford I, Linnoila M. Early-onset alcoholics have lower cerebrospinal fluid 5-hydroxyindoleacetic acid levels that late-onset alcoholics. Arch Gen Psychiatry. 1996. 53:211–216.
75. Swann AC, Johnson BA, Cloninger CR, Chen YR. Relationships of plasma tryptophan availability to course of illness and clinical features of alcoholism: a preliminary study. Psychopharmacology(Berl). 1999. 143:380–384.
76. Book SW, Myrick H. Novel anticonvulsants in the treatment of alcoholism. Expert Opin Investig Drugs. 2005. 14:371–376.
77. Johnson BA, Ait-Daoud N, Akhtar FZ, Ma JZ. Oral topiramate reduces the consequences of drinking and improves the quality of life of alcohol-dependent individuals: a randomized controlled trial. Arch Gen Psychiatry. 2004. 61:905–912.
78. Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003. 361:1677–1685.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download