Abstract
Inflammatory pseudotumor (IPT) of the liver is rare benign tumor. When the diagnosis of IPT is established with biopsy, simple observation or conservative therapy is preferred because of the possibility of regression. But IPT is unresponsive to the conservative treatment, surgical resection should be considered. We experienced a 63-year-old male, who was suspected hepatocellular carcinoma in abdominal computed tomography (CT) and magnetic resonance image (MRI) scan, presented with 2-month history of intermittent fever and weight loss. Percutaneous ultrasound guided core biopsy confirmed IPT of the liver. Non-steroidal anti-inflammatory drugs and antibiotics were administered for 8 and 4 weeks, respectively, but fever continued. So, extended right hepatectomy was performed for IPT of the liver and then fever subsided. The patient remains well during a follow-up period of 12 months.
Inflammatory pseudotumors (IPT) have been reported in the lung, liver, orbit, mediastinum, bronchus, small intestine, mesentery, kidney, spleen, stomach, meninges, spine, thyroid gland, and urinary bladder.1-3
IPT of the liver, first described by Pack and Baker,4 is a rare lesion which is frequently confused with malignant tumors. Torzilli et al.5 have reported three cases (0.7%) of IPT that were found among 403 patients who had undergone liver resection. In this decade, the incidence of IPT is increasing because of the improvements on computed tomography (CT) scan and the development of percutaneous ultrasound guided core biopsy. After the diagnosis of IPT of the liver, conservative therapy is advocated for the initial treatment.6 Surgical treatment should be considered when the IPT does not respond to conservative therapy.
We herein report a case of IPT of the liver that was unresponsive to conservative therapy, and the patient was then treated by hepatic resection.
A 63-year-old man was referred to our hospital on April 23, 2003 because of the findings on abdominal CT and a MRI scan that were taken at a local community hospital. The findings showed a single liver tumor measuring 6 cm in diameter in the right lobe (Fig. 1). He presented with a two-month history of intermittent fever that ranged from 37.5 to 38.0℃ and a weight loss of 4 kg (64 kg to 60 kg). The physical examination was unremarkable.
Laboratory investigations revealed that the hemoglobin level was 9.3 g/dL, and the white blood cell count was 8,680/µL with segmental neutrophilia (78.2%). There was no eosinophilia (1.4%). The erythrocyte sedimentation rate was elevated to 97 mm/h (normal range : 0-15 mm/h) and the serum C-reactive protein level was also elevated to 8.18 mg/dL (normal range : 0-0.8 mg/dL). The liver function tests were within normal limits as were the tests for serum alpha-fetoprotein (αFP), carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9. Serology for hepatitis A, B, or C was negative. No organisms were identified on the cultures of peripheral blood. Intradermal skin tests for Clonorchis sinensis and Paragonimus westermani were negative.
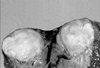
Percutaneous ultrasound guided core biopsy was performed to allow a histological diagnosis of the liver mass, and it confirmed IPT of the liver (Fig. 2). Cultures of biopsed tissue were not done.
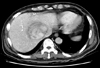
The patient was initially treated with antibiotics (cefpiramide, amikacin, metronidazole, teicoplanin and, imipenem) for 4 weeks and a nonsteroidal anti-inflammatory drug (Naproxen) was prescribed for 8 weeks. Yet the fever continued to spike to 38.0℃ and the lesion still remained on the follow-up CT scan that was done July 13, 2003 (Fig. 3). Therefore extended right hepatectomy was carried out on July 16, 2003, and a tumor, 6 cm in diameter, was found in Couinaud's segment VIII of the liver (Fig. 4). The histologic findings were consistent with the features of IPT (Fig. 5). There were no complications and the fever subsided during the postoperative period. The patient was discharged on the 14th postoperative day, and he is now doing well without any recurrent fever during the follow-up period of 12 months.
The exact pathogenesis for IPT of the liver has not yet been well characterized, but the inflammatory pathological pattern and the systemic symptoms including fever and malaise suggested there was an underlying infectious agent. However, in many reports, no causative microorganisms have been identified in the blood cultures, the same as was noted in our case.7
The differential diagnosis from malignant tumor can be difficult, because the radiologic findings of IPT are rather nonspecific.8 Percutaneous biopsy is most reliable method, and it enables us to avoid unnecessary exploratory laparotomy or a hepatectomy when there is an uncertain diagnosis. On rare occasion, an occult adenocarcinoma could be mistaken as an IPT.9 Pseudotumor should be kept in mind in the differential diagnosis of malignant liver lesions.
Numerous studies have shown that the natural history of IPT is one of disease regression. Once the diagnosis of IPT has been confirmed with biopsy, patients with IPT can simply be observed and regular follow-up is performed until the condition resolves itself, or the patient can be medically treated with antibiotics, anti-inflammatory drugs and steroid.10
Although surgical resection is generally regarded as excessive treatment, surgery should be considered in the following situations. The first case is that the systemic symptoms with fever do not resolve in spite of conservative therapy, as was the situation in our case.11 The second situation is that IPT is noted to grow, with or without symptoms, on serial examinations and the imaging studies.12 The last situation is IPT that involves the hepatic hilum, and thic can causes biliary obstruction and portal hypertension.13
Although symptomatic patients may benefit from steroid administration, we selected liver resection as the treatment of choice instead of steroid therapy in this case because there are no clinical reports showing that steroid therapy promotes resolution of IPT.10 For respectable IPTs that are unresponsive to antibiotics, surgery could be a better option rather than steroid therapy or simple observation.
Figures and Tables
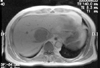 | Fig. 1Contrast enhanced T1 weighted MRI scan shows a relatively well-encapsulated, hypervascular mass, 6 cm in diameter on the right lobe of the liver. |
 | Fig. 2Percutaneous biopsy shows the marked infiltration of lymphoplasma cells and some lymphocytes that are without cytologic atypia (H&E, × 400). |
References
1. Broughan TA, Fischer WL, Tuthill RJ. Vascular invasion by hepatic inflammatory pseudotumor. A clinicopathologic study. Cancer. 1993. 71:2934–2940.
2. Byun YJ, Chung BH, Kwon KW. Inflammatory pseudotumor of urinary bladder. Yonsei Med J. 2000. 41:273–275.
3. Choi SK, Choi YD, Cheon SH, Byun Y, Cho SW. Inflammatory pseudotumor of the urinary bladder in a child. Yonsei Med J. 2000. 41:401–403.
4. Pack GT, Baker HW. Total right hepatic lobectomy: report of a case. Ann Surg. 1953. 138:253–258.
5. Torzilli G, Inoue K, Midorikawa Y, Hui AM, Takayama T, Makuuchi M. Inflammatory pseudotumors of the liver: prevalence and clinical impact in surgical patients. Hepatogastroenterology. 2001. 48:1118–1123.
6. Jais P, Berger JF, Vissuzaine C, Paramelle O, Clays-Schouman E, Potet F, et al. Regression of inflammatory pseudotumor of the liver under conservative therapy. Dig Dis Sci. 1995. 40:752–756.
7. White JE, Chase CW, Kelley JE, Brock WB, Clark MO. Inflammatory pseudotumor of the liver associated with extrahepatic infection. South Med J. 1997. 90:23–29.
8. Nam KJ, Kang HK, Lim JH. Inflammatory pseudotumor of the liver: CT and sonographic findings. AJR. 1996. 167:485–487.
9. Schwarz RE, Reynolds JC, Lotze MT. "Pseudo-pseudotumor" of the liver. Adenocarcinoma presenting as an inflammatory pseudotumor. Dig Dis Sci. 1994. 39:2679–2684.
10. Koea JB, Broadhurst GW, Rodgers MS, McCall JL. Inflammatory pseudotumor of the liver: demographics, diagnosis, and the case for nonoperative management. J Am Coll Surg. 2003. 196:226–235.
11. Gluszek S, Kot M, Czerwaty M. Inflammatory pseudotumor of the liver treated surgically. Hepatogastroenterology. 1999. 46:2959–2960.
12. Lu CC, Chen CC, Hsia CY, Chiang JH, Tsay SH, Han HF, et al. A progressive growing inflammatory pseudotumor of the liver. Zhonghua Yi Xue Za Zhi (Taipei). 2001. 64:725–730.
13. Kaneko K, Ando H, Watanabe Y, Seo T, Nagino M, Kamiya J, et al. Aggressive preoperative management and extended surgery for inflammatory pseudotumor involving the hepatic hilum in a child. Surgery. 2001. 129:757–760.
14. Pecorella I, Ciardi A, Memeo L, Trombetta G, de Quarto A, de Simone P, et al. Inflammatory pseudotumour of the liver-evidence for malignant transformation. Pathol Res Pract. 1999. 195:115–120.
15. Zavaglia C, Barberis M, Gelosa F, Cimino G, Minola E, Mondazzi L, et al. Inflammatory pseudotumour of the liver with malignant transformation. Report of two cases. Ital J Gastroenterol. 1996. 28:152–159.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download