Abstract
The purpose of this study was to demonstrate corticospinal tract compression that was due to a hematoma by using diffusion tensor tractography (DTT) and functional MRI (fMRI) in a patient with an intracerebral hemorrhage (ICH). A 23-year-old right-handed woman presented with severe paralysis of her right extremities at the onset of a spontaneous ICH. Over the first three days from onset, the motor function of the affected upper and lower extremities rapidly recovered to the extent that she was able to overcome applied resistance to the affected limbs, and her limbs regained normal function 3 weeks after onset. The tract of the right hemisphere originated from the primary sensori-motor cortex (SM1) and it passed through the known corticospinal tract pathway. However, the tract of the left hemisphere was similar to that of the right hemisphere except that it was displaced to the antero-medial side by the hematoma at the cerebral peduncle. Only the contralateral SM1 area centered on the precentral knob was activated during affected (right) or unaffected (left) hand movements, respectively. In conclusion, fMRI and DTT demonstrated a corticospinal tract compression due to hematoma in this patient. We conclude that the combined use of these two modalities appears to improve the accuracy of investigating the state of the corticospinal tract.
The putative motor recovery mechanisms following a stroke involve contributions from the unaffected motor cortex, the secondary motor area, the cortical territory adjacent to the lesion and the contralateral corticospinal tract.1-3 An understanding of the motor recovery mechanisms has important implications for brain rehabilitation because it may provide the information required for estimating the prognosis and for establishing effective rehabilitation strategies.
Diffusion tensor tractography (DTT) is a recently introduced technique that can visualize the architecture and integrity of the white matter tracts.4-8 Conversely, functional MRI (fMRI) can precisely localize the activation sites due to its excellent spatial resolution at the cortex level.9 Thus, it seems that the fusion of these two modalities would allow for more accurate investigations of the motor pathway.
In the current study, we herein report upon a patient having an intracerebral hemorrhage (ICH) with a compressed corticospinal tract, and this was determined by combined DTT and fMRI.
A 23-year-old right-handed woman presented with complete paralysis of the right extremity at the onset of a spontaneous ICH. T2-weighted MR images showed a hematoma in the left cerebral peduncle (Fig. 1).
Three days after the onset, the motor function of the affected upper and lower extremities rapidly recovered to the extent of her being able to actively overcome applied resistance to her limbs. At the third week from the onset, she was able to perform fine motor activities, such as writing and using chopsticks with her affected (right) hand, and she could walk with a normal gait; she also had normal grip power (13 kg, normal range: 14.1 kg ± 9.5) and normal fine motor ability (Purdue pegboard test: 16, normal range: 17.4 ± 1.6) at that time.10 The patient signed an informed consent statement for the present study, and the study protocol was approved by our Institutional Review Board prior to the study's commencement. DTT and fMRI were performed 3 weeks after the onset of her symptoms.
All studies were performed using a 1.5-T Philips Gyroscan Intera system equipped with a synergy-L Sensitivity Encoding (SENSE) head coil. The diffusion tensor images were acquired using a single-shot spin echo-planar imaging sequence with two diffusion-sensitizing gradients before and after a 180° radio-frequency pulse, and navigator echo-phase correction was employed for motion correction. For each of the 32 noncollinear diffusion-sensitizing gradients, we acquired 60 contiguous slices parallel to the anterior commissure-posterior commissure line. The imaging parameters used were: matrix = 128 × 128, field of view = 221 × 221 mm2, TE = 76 ms, TR = 10,726 ms, SENSE factor = 2, EPI factor = 67 and b = 600 mm2 s-1, NEX = 4, and a 2.3 mm slice thickness.
A substantial amount of interest is now being shown concerning fiber tracking methods and several different approaches are currently being used. In the present study, 3-D reconstructions of the fiber tracts were obtained using the PRIDE software package (Philips Medical Systems, Best, Netherlands) based on fiber assignments that were done by the continuous tracking (FACT) algorithm.4,11 The termination criteria used were FA < 0.3, and the angle > 45°. In our study a seed region of interest (ROI) was drawn in the blue portion of the upper anterior pons on 2-D FA color maps: colors were assigned as follows: red (x component, left-right), green (y component, anterior-posterior), and blue (z component, superior-inferior). A target ROI was drawn in the blue portion of the anterior medulla on the 2-D color map. Fiber tracts passing through both ROIs were designated as the final tracts of interest. 3-D fiber tracts were then superimposed on the T2-weighted axial images.
The tract from the right hemisphere originated from the primary sensori-motor cortex (SM1) and it consecutively passed through the corona radiata, the posterior limb of the internal capsule, the cerebral peduncle of the midbrain, the anterior pons and the anterior medulla along the known corticospinal tract pathway (Fig. 2). The tract of the left hemisphere followed a similar course except that it was displaced to the antero-medial side by a hematoma at the cerebral peduncle (Fig. 2-arrow).
The patient was examined in the supine position, and she firmly secured using an immobilizing frame with her forearms in a prone position and with her eyes closed. For the motor task paradigm, she performed hand grasp-release movements at a frequency of 1 Hz for stimulation, guided by a metronome, over a repeated cycle of 21 seconds of control and 21 seconds of stimulus. Each 42-second task paradigm of alternating control-stimulus (42 seconds) was repeated three times.
Using the Echo Planar Imaging (EPI) technique, blood oxygenation level dependent (BOLD) fMRI measurements were taken using a 1.5T MR scanner (Vision, Siemens, Erlangen, Germany) that was fitted with a standard head coil. For anatomic base images, 20 axial, 5-mm thick, T1-weighted, conventional, spin echo images were obtained with a matrix size of 128 × 128 and a field of view (FOV) of 210 mm parallel to the bicommissure line of the anterior commissure-posterior commissure (AC-PC). EPI BOLD images were acquired over the same 20 axial sections for each epoch, producing a total of 1200 images for this patient. The imaging parameters used were; TR/TE = 2 sec/6 msec, FOV = 210 mm, matrix size = 64 × 64 and slice thickness = 5 mm. The fMRI data obtained was analyzed using SPM-99 software (Wellcome Department of Cognitive Neurology, London, UK) running under the MATLAB environment (Mathwork, Inc., Natick, Ma, USA). The images were smoothed using an 8 mm isotropic Gaussian kernel. Statistical parametric maps were obtained and voxels were considered significant at a threshold of p < 0.05 (corrected), with an additional requirement set for a minimum cluster size of 5 voxels.
Only the contralateral SM1 was activated during the affected (right) or unaffected (left) hand movements, respectively (Fig. 3).
According to our results from our brain scans, it seems that in this case the corticospinal tract was compressed by a hematoma in the cerebral peduncle. First, the patient showed a rapid motor recovery over a period of 3 days after onset. This strongly suggests that the motor function recovered after the resolution of local factors such as hematoma or edema, and her recovery was not due to a process of brain plasticity.12 Second, fMRI showed that only the contralateral SM1 centered on the precentral knob, which is known to be the neural center for the hand,13 was activated by movements of either hand. Moreover, the tracts originating from both hemispheres descended along the known pathway of the corticospinal tract, except for a deviation at the left cerebral peduncle. However, the tract for the left hemisphere was small compared to that of the right. We believe that this phenomenon was caused by partial corticospinal tract damage due to the hematoma.
The corticospinal tract is essential for good motor recovery following stroke because it is mandatory for proper motor function in humans.14-16 Therefore, elucidating the status of the corticospinal tract has important implications because this can provide information the physicain requires for estimating the prognosis for stroke patients. Visualization of the corticospinal tract has previously not been possible in the human brain because popularly used brain mapping techniques such as functional neuroimaging or transcranial magnetic stimulation cannot visualize the corticospinal tract. In the current study, combined DTT and fMRI techniques were used to demonstrate corticospinal tract compression by a hematoma. Therefore, it seems that the combined use of these modalities would allow for more accurate investigations of the corticospinal tract.
Figures and Tables
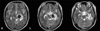 | Fig. 1T2-weighted images showing a hematoma in the left cerebral peduncle. T2-weighted images showing a hematoma in the left mid to lateral portion of the cerebral peduncle. |
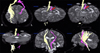 | Fig. 2Results of 3-D diffusion tensor tractography. The tract from the right hemisphere originated in the primary sensori-motor cortex, and it consecutively passed through the corona radiata, the posterior limb of the internal capsule, the cerebral peduncle of the midbrain, the basis pontis, and the anterior medulla along the known corticospinal tract pathway. The tract of the left hemisphere followed a similar course except that it was displaced to the antero-medial side due to the hematoma at the cerebral peduncle, and it was also smaller than the right hemisphere tract (red arrow). |
References
1. Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997. 28:2518–2527.
2. Jang SH, Cho SH, Kim YH, Kwon YH, Byun WM, Lee SJ, et al. Cortical activation changes associated with motor recovery in patients with precentral knob infarct. Neuroreport. 2004. 15:395–399.
3. Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke. 2003. 34:1553–1566.
4. Mori S, van Zijl PC. Fiber tracking: principles and strategies - a technical review. NMR Biomed. 2002. 15:468–480.
5. Yamada K, Mori S, Nakamura H, Ito H, Kizu O, Shiga K, et al. Fiber-tracking method reveals sensorimotor pathway involvement in stroke patients. Stroke. 2003. 34:E159–E162.
6. Lee SK, Mori S, Kim DJ, Kim SY, Kim SY, Chu M, et al. Diffusion tensor MRI and fiber tractography of cerebellar atrophy in phenytoin users. Epilepsia. 2003. 44:1536–1540.
7. Lee SK, Mori S, Kim DJ, Kim SY, Kim SY, Kim DI. Diffusion tensor MR imaging visualizes the altered hemispheric fiber connection in callosal dysgenesis. Am J Neuroradiol. 2004. 25:25–28.
8. Kim YH, Jang SH, Han BS, Kwon YH, You SH, Byun WM, et al. Ipsilateral motor pathway confirmed by diffusion tensor tractography in a patient with schizencephaly. Neuroreport. 2004. 15:1899–1902.
9. Macdonell RA, Jackson GD, Curatolo JM, Abbott DF, Berkovic SF, Carey LM, et al. Motor cortex localization using functional MRI and transcranial magnetic stimulation. Neurology. 1999. 53:1462–1467.
10. Kim YT, Kang SY, Kim HS, Shin BS. Hand strength and dextricity evaluation with age. J Korean Acad Rehab. 1994. 18:780–788.
11. Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999. 45:265–269.
12. Witte OW. Lesion-induced plasticity as a potential mechanism for recovery and rehabilitative training. Curr Opin Neurol. 1998. 11:655–662.
13. Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997. 120:141–157.
14. Bastings EP, Rapisarda G, Pennisi G, Noordhout AM, Lenaerts M, Good DC, et al. Mechanisms of hand motor recovery after stroke: an electrophysiologic study of central motor pathways. J Neuro Rehabil. 1997. 11:97–108.
15. Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001. 21:5272–5280.
16. Binkofski F, Seitz RJ, Arnold S, Classen J, Benecke R, Freund HJ. Thalamic metabolism and corticospinal tract integrity determine motor recovery in stroke. Ann Neurol. 1997. 39:460–470.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download