INTRODUCTION
Soft tissue myoepithelial tumors were only recently recognized to occur primarily and rarely in soft tissue and skin and their clinicopathologic characteristics has been limited.1 The occurrence of the soft tissue myoepithelioma is very rare in the head and neck region.1,2 So far, only one case of soft tissue myoepithelial tumor occurring in the masticator space has been reported in the world literature.3 Herein, a case of soft tissue myoepithelial tumor with benign histomorphology, but with an invasive growth pattern, occurring in masticator space was presented.
CASE REFORT
Clinical summary
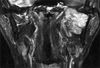
A 46-year-old male patient was admitted due to painful swelling on the left mandibular area lasting for several months. Past and family histories were unremarkable. MR PNS nasopharynx (coronal T2 weighted image) revealed a well defined, well enhancing heterogeneous mass with high signal intensity and dense calcification in the masticator space between the left mandible ramus and pterygoid process (Fig. 1). This mass measured 6 × 5.5 cm and had a lobulated margin. Adjacent bony structure, muscle and parapharyngeal space were well preserved. Partial mandibulectomy was performed.
Pathologic findings
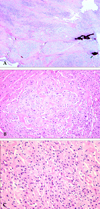
Grossly, the tumor was a well-circumscribed ovoid solid mass and consisted of yellowish gray glistening firm tissue. A fragment of bone tissue was attached on one side. Histologically, the tumor showed a multinodular growth pattern (Fig. 2A). The tumor was composed of epithelioid cells in chondromyxoid stroma (Fig. 2B) and of spindle-shaped to ovoid cells in the hyaline stroma (Fig. 2C). The epithelioid round cells were isolated or in small groups and had an eosinophilic or clear cytoplasm with eccentric nuclei. The nucleoli were not prominent, and the tumor cell margins were distinct from each other. The spindle cells had bland-looking nuclei and an indistinct eosinophilic cytoplasm and were randomly arranged. Neither mitosis nor necrosis was found. Although the tumor for the most part was well circumscribed in both the gross and histological perspectives, it focally invaded to adhered bone tissue.
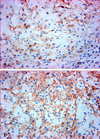
Immunohistochemically, the tumor cells were diffusely positive for epithelial membrane antigen (EMA) (Fig. 3A), but negative for other epithelial markers (Pan-cytokeratin, CAM 5.2, high MW-cytokeratin, CK 7 and CK 20). The tumor was diffusely positive for vimentin, smooth muscle actin (Fig. 3B) and focally positive for S-100 protein, but was negative for glial fibrillary acidic protein (GFAP), desmin, and CD34.
The electron microscopic examination revealed ovoid tumor cells that were widely separated by an abundant collagenous matrix. Their nuclei had indentations, finely dispersed chromatin, and occasional small nucleoli. The cytoplasm contained sparse microfilaments and subplasmalemmal densities (Fig. 4A), and attenuated desmosomes were commonly seen (Fig. 4B). On the other hand, basal lamina was not discernable.
DISCUSSION
Well-characterized in salivary glands, myoepitheliomas were only recently recognized to occur primarily yet rarely in soft tissue and skin, and their characterization, especially their clinical behavior and clinicopathologic correlation, to date has been limited.1 Histopathologic diagnosis is based on the finding of neoplastic cells bearing close resemblance to the myoepithelial cells of pleomorphic adenoma of salivary glands.4 In soft tissue myoepithelioma, the diagnosis was confined to the tumors that were either lacking or with very limited ductal differentiation and soft tissue myoepithelioma was considered to be in morphologic continuum with pleomorphic adenoma,1 which is the same as the counterparts of the salivary gland and skin.
The neoplastic myoepithelial cells can display a wide range of morphologic features,1 such as spindle-shaped, ovoid, plasmacytoid, hyaline, epithelioid, or clear cells.4 In addition, neoplastic stroma is also variable, and it may appear myxoid or shows different forms of mesenchymal metaplasia.4 Variations in cellularity, cytomorphology, abundance of stroma, and occasional foci of epithelial or mesenchymal metaplasia results in the extremely heterogeneous appearance of these neoplasms,1 making histopathologic diagnosis rather difficult.4
Myoepithelial cells possess the capacity for epithelial and myoid differentiation, and as a consequence, immunohistochemical reactivity is also variable.4 Ultrastructurally, the myoepithelial cells have a basal membrane with desmosomes, a large number of cytoplasmic filaments, pinocytic vesicles and dense body formation.4
The absence of clear-cut histopathologic clues for the diagnosis of myoepithelioma is hampered further by the wide variability in their immunohistochemical characteristics. Immunoreactivity for S-100 and muscle actin seems to be the most constant immunophenotype, whereas immunoexpression for epithelial markers, such as cytokeratin and EMA, or neural markers, such as GFAP, is somewhat erratic and variable.4 Neoplastic myoepithelial cells (especially spindle cells) are occasionally cytokeratin negative but show strong expression of EMA instead.5 In a study of cutaneous myoepithelioma, EMA was detected in most tumors, whereas positivity for keratin is seen in 64% of tumors. Pan-cytokeratin, AE1/AE3, and CK8/18 each stain approximately 50% of cutaneous myoepithelioma.6 Also, the neoplastic myoepithelial cells often lose the ability to express myogenic markers, and hence, such immunostaining may not be required to confirm myoepithelial differentiation.6 Therefore, immunostaining is required for keratin and/or EMA, in conjunction with S-100 protein or GFAP and myogenic markers, to confirm the diagnosis of myoepithelioma.1,6 Therefore, the present case can be diagnosed as myoepithelial tumor because the tumor cells were diffusely positive for EMA and actin, irrespective of the negative results for several cytokeratin antibodies.
Investigation by electron microscopy is exceptional in soft tissue myoepithelioma. Ultrastructural features of soft tissue myoepitheliomas are similar to those of their analogous counterpart of salivary glands, which exhibit variable contents of intermediate filaments of the vimentin type, focal densities resembling smooth muscle dense bodies, pinocytic vesicles, some desmosomes with tonofibril like bundles, and foci of basal lamina.7 This case illustrated microfilaments with subplasmalemmal densities and desmosomes, and thus, it confirms the myoepithelial origin of the tumor.
The differential diagnosis in the present case includes extraskeletal myxoid chondrosarcoma (EMC) and ossifying fibromyxoid tumor (OFMT). In contrast to EMC, the cells in myoepithelioma are generally more epithelioid with distinct cell margins,6 and epithelial and myogenic markers are rarely seen in EMC. OFMT is a lobulated tumor composed of round to ovoid cells in a hyalinized or myxoid stroma, usually with a peripheral rim of metaplastic bone. S-100 protein is often expressed in OFMT, and about 50% of this tumor is also positive for desmin. In contrast, cutaneous myoepitheliomas are usually negative for desmin, and nearly always positive for epithelial markers.6 Therefore, the possibilities of EMC and OFMT can be ruled out in the present case based on immunohistochemical results, as well as histologic features. Finally, the possibility of ectomesenchymal chondromyxoid tumor (ECT) can be considered in this case, because ECT and myoepithelial tumors had similar histologic and immunohistochemical findings. However, ECT exclusively occurred in the tongue, and features of myoepithelial cells, such as desmosomes and condensations of thin filaments, were not seen in the electron microscopy.8,9
Soft tissue myoepitheliomas are most common in the limb and limb girdles.1 The occurrence in the head and neck region is very rare with soft tissue myoepithelioma.1,2 So far, only one case of soft tissue myoepithelial tumor occurring in the masticator space has been reported in the world literature.3 That tumor was composed of spindle cells undergoing frequent mitosis, and the tumor metastasized to the lung and arm and ultimately caused patient's death 32 months after the initial diagnosis despite of radiotherapy and chemotherapy.3 Because of the lack of myoepithelial cells in the masticator space, myoepitheliomas arising in the masticator space can be regarded as soft tissue origin.3 The present case was also considered as a soft tissue myoepithelial tumor, because adjacent bony structure, muscle, and parapharyngeal space were well preserved.
For salivary gland myoepitheliomas, in the absence of frankly malignant cytomorphology, the histological feature reported to indicate malignancy included infiltration of surrounding tissue and a high mitotic rate.6 However, no specific mitotic rate cutoff exists for making this distinction. Thus, in the absence of flank malignant cytomorphology, an invasive growth pattern is the single most useful criterion for establishing malignancy in salivary myoepithelioma.1 The malignant criteria for soft tissue myoepithelioma are still controversial.10 In soft tissue myoepithelioma, the only histological feature that is significantly associated with recurrence or metastasis is the presence of moderate to severe cytologic atypia, and infiltrative borders and mitotic rate do not correlate with aggressive behavior in contrast to the salivary myoepithelial tumor.1 Tumors with benign or low-grade cytology did not undergo metastasis and none of these patients died of disease, although a few cases recurred locally.1 In a large series of myoepithelial tumors, about half of the tumors showed at least focal infiltration into the surrounding tissues, and only 2 cases invaded bone.1 No clinical or pathologic parameters, including patient age, status excision margins, tumor depth, tumor size, infiltration to surrounding tissues, presence of tumor necrosis, and mitotic rate, correlated with recurrence or metastasis.1 However, a case of histologically benign cutaneous myoepithelioma metastasized to a submaxillary lymph node,6 and this tumor showed a high mitotic rate.
The present tumor showed a histologically benign appearance, but invaded to adhered bone tissue, which was not correlated with aggressive clinical behavior according to previous reports of soft tissue myoepithelial tumors. Until additional reports and larger series of soft tissue myoepithelial tumors are available, it is prudent to advise, in reference to these tumors, complete excision and appropriate clinical follow-up study,4 including the present case.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download