Abstract
Primary splenic lymphoma (PSL) is often defined as generalized lymphoma with splenic involvement as the dominant feature. It is a rare disease that comprises approximately 1% of all malignant lymphomas. We investigated three cases of non-Hodgkin's splenic lymphoma that had different clinical features on presentation. The patients' survival times from diagnosis ranged from 59 to 143 months, without evidence of relapse after splenectomy and chemotherapy, with or without radiotherapy. This data suggest that PSL is potentially curable. Further studies are needed to evaluate the impact that different treatment modalities without splenectomy have on patient survival.
Generalized lymphoma with splenic involvement as the dominant feature is known as primary splenic lymphoma (PSL); however, this definition is controversial. PSL is a rare disease with only 1% of all malignant lymphomas reported to be PSL. Additionally, there have been very few reported cases in Korea.1,2 However, about 20% of all lymphoma patients seen at our hospital have had evidence of splenic involvement.3
In this study, we report three cases of PSL with variable clinical presentations and the excellent results different therapies had on theses patients' survival times.
A 58-year-old woman presented on July 8, 1997 with indigestion for 20 days and left upper quadrant (LUQ) pain for one week. She had taken antihypertensive medications for two years. Her examination was significant for direct LUQ tenderness and absence of fever, night sweats, weight loss, organomegaly, or lymphadenopathy. Significant laboratory data included an elevated lactate dehydrogenase (LDH) level of 180 IU/dL (reference value 56-123 IU/dL) with an elevated LD 3, 4 fraction, and a β2-microglobulin level of 3.4 mg/dL. Esophagogastroduodenoscopy revealed a fundal mass or external compression of the fundus, and a barium study of the upper gastrointestinal tract showed an external mass. Computed tomography (CT) of the abdomen showed a huge splenic mass with a homogenous high-density area, including a portion of low-density without evidence of perisplenic spread (Fig. 1A). A benign splenic tumor was suspected and the patient underwent splenectomy. The spleen weighed 442 g and measured 12.5 × 8 × 7 cm. On gross examination, a grayish-yellow nodular capsule was noted in the lateral border without perisplenic invasion or any enlarged hilar lymph nodes. The exposed cut surface showed a bulging, well-demarcated grayish-yellow nodular solid mass, measuring 12 cm in the largest dimension (Fig. 2). Histopathology confirmed diffuse large B-cell lymphoma (Fig. 3A, B) with positive expression of L26/CD79a (Fig. 3C), and no expressions of UCHL1, CD3, and granzyme upon immunohistochemical staining.
Neck & chest CT scanning and whole body bone scanning revealed no abnormalities. Bone marrow aspiration & biopsy did not reveal any evidence of lymphoma. The lymphoma was staged at Ann Arbor stage ISEA and Ahmann stage I. The patient received six cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy, and has been in complete remission without relapse for 88 months.
A 53-year-old man was admitted in December 1992 with intermittent left flank discomfort that had persisted for four months. He had a history of pulmonary tuberculosis treated with anti-tuberculosis medications 30 years ago. He was relatively well until four months prior to admission, when the left flank and abdominal discomfort developed. No significant findings were noted on physical examination. Laboratory findings showed no cytopenia, normal levels of LDH and β2-microglobulin, but an elevated ESR (erythrocyte sedimentation rate, Westergren: 24 mm/hr). An abdominopelvic CT scan revealed a large, relatively homogenous hypo-attenuating lesion (measuring 8×7×9 cm) in the LUQ abutting the pancreatic tail & inferior pole of the spleen. This lesion was localized and capsulated with either internal necrosis or hemorrhage (Fig. 1B). Several nodal areas of about 1 cm in diameter were noted in the left para-aortic area slightly above the left renal hilar level. Neck and chest CT scans and a whole body bone scan were normal. Splenectomy, distal pancreatectomy, and segmental resection of the transverse colon revealed a large irregular oval shaped mass (9×8×7 cm) in the enlarged spleen (13×10×8 cm) and tail of pancreas (3×2.5×2 cm), with the spleen weighing only 570 g. The mass adhered to the transverse colon. The Ann Arbor stage was IISEA and the Ahmann stage of splenic lymphoma was III. Histology of the resected spleen, pancreas, and colon revealed non-Hodgkin's lymphoma of the diffuse large B-cell type. Postoperatively, the patient received six cycles of CHOP chemotherapy and radiotherapy to the left abdomen (total dose of 3,060 cGy). While in complete remission, he visited the emergency room in October 1993 with a left ureteral stone attack, which was relieved after conservative treatment. He was noted to be in complete remission at the time of follow-up, 143 months after diagnosis.
A 52-year-old woman was admitted in December 1999 with a 6-month history of anorexia and general weakness. She had previously visited a hospital in Brazil where she underwent an abdominal CT scan, which showed a splenic mass. She then came to Korea for further evaluation and treatment.
Twenty-eight years prior to admission, she underwent dilatation & evacuation three times for a hydatidiform mole. Twenty-seven years prior to admission, she was diagnosed with pulmonary tuberculosis and she received anti-tuberculosis medications. Twenty-six years before admission, she was diagnosed with choriocarcinoma and she underwent total abdominal hysterectomy with adjuvant chemotherapy.
At the time of admission, she complained of generalized weakness, fatigue, chills, sore throat, night sweats, mild fever, productive cough, and poor oral intake but denied weight loss. Multiple small cervical lymphadenopathy without splenomegaly was detectable. Significant laboratory data included a hemoglobin level of 9.6 g/dL, hematocrit of 28%, total leukocyte count of 7,900/uL (differential: neutrophils, 57%: lymphocytes, 23%: monocytes, 19%: eosinophil, 1%), and a platelet count of 83,000/uL. The CT scan of the abdomen taken in Brazil revealed a heterogeneous lobulated splenic mass measuring 10×8×8 cm with bulging contours, necrotic foci, and focal calcification (Fig. 1C). Angiosarcoma, malignant lymphoma, and metastatic tumor were considered in the differential diagnosis. Bone marrow aspiration and biopsy revealed no abnormal cell infiltration.
The patient then underwent splenectomy for diagnosis. Intra-operatively, the spleen was found to be enlarged and congested, and its cut surface was grayish-yellow with focal necrosis. There was a 1-cm sized accessory spleen that was also resected. Gross pathology revealed a lobulated grayish-yellow solid mass with a homogenous cut surface resembling fish flesh with focal necrosis. The spleen measured 12×11×6.5 cm, and weighed 507 g. The pathologic diagnosis was diffuse large B-cell lymphoma, as the tumors were positive for B-cell marker (L26/CD79a) and negative for T-cell marker (CD3). Neck CT depicted bilateral small lymphadenopathy along the internal jugular chain, submental area, and level II area of the neck, measuring 1.8×1.1 cm in maximal diameter. Four days after splenectomy, the patient's platelet counts returned to a normal level of 333,000/uL. She was staged at Ann Arbor stage IIISEB and Ahmann stage III. After six cycles of ProMACE-CytaBOM (cyclophosphamide, adriamycin, VP16, prednisone, cytarabine, bleomycin, vincristine and methotrexate) chemotherapy, the patient returned to Brazil and was found to be in complete remission at the time of follow-up, 59 months after diagnosis.
The spleen serves as a complex filter to clear the blood of particulate matter and senescent blood cells. This organ normally weighs about 100-250 g (average, 135 g) and usually measures 12 cm in length, 7 cm in width, and 3 cm in thickness.4-6
The spleen is also involved in immune defense against blood-borne antigens.4 More than half of patients affected by Hodgkin's disease (HD) and about a third of those with non-Hodgkin lymphoma (NHL) have splenic involvement.7 The splenic weight of primary splenic lymphoma (PSL) patients is usually within the range of 226-4,000 g, according to a report by Kraemer et al.8 In this study the average splenic weight was found to be 506.3 g (range, 442-570 g). Splenic involvement can be a part of diffuse dissemination of NHL, in which the spleen is one of many involved organs. Alternatively, non-Hodgkin's lymphoma may also originate in the spleen and then spread to other sites.8-10
The definition of PSL is controversial; it can be summarized with three different definitions. Das Gupta et al. adopted a restrictive definition of PSL as a lymphoma involving only the spleen and the splenic hilar lymph nodes.11 According to this definition, the diagnosis of PSL can only be made if isolated splenomegaly occurs in the absence of any other localized tumors, particularly in the liver or the para-aortic or mesenteric lymph nodes. Before confirming the diagnosis of PSL, the authors recommend that a 6-month relapse-free period should exist after removal of the spleen. On the other hand, Skarin et al. suggested that the diagnosis of PSL can be made if splenomegaly is a predominant feature in any lymphoma involving the spleen.9,12,13 On the other hand, Kraemer et al. reserved the diagnosis of PSL for patients with splenomegaly, cytopenia of at least two hematologic cell lines, and the absence of peripheral adenopathy.8 In a report by Kehoe et al., the authors mentioned that Das Gupta's conditions defined early and localized disease. These conditions did not adequately account for other PSL patients with more advanced disease or disease discovered later in its course.9 Kehoe et al. also pointed out that a built-in survival advantage bias existed in Das Gupta's patients and thus chose to define PSL as NHL arising primarily in the spleen or as NHL principally confined to the spleen and its local lymph nodes.9 In our report, the first two cases are consistent with PSL with all three definitions noted. However, in the third case, due to multiple cervical lymphadenopathies, the diagnosis was made using the definition by Skarin et al. Although there cervical lymphadenopathy was present, we accepted it as PSL because the dominant tumor burden was in the spleen and given the possibility of spread from it.8 In addition, the possibility that the cervical lymphadenopathy had an infectious origin cannot be ruled out, in view of such symptoms on admission as productive cough and sore throat, even if there was no pathologically confirmed lymphoma.
Symptoms of PSL include fever, weight loss, generalized weakness, and LUQ pain or discomfort from splenomegaly. There are also other specific symptoms that result from direct invasion of the pancreas, stomach, diaphragm, colon, or greater omentum.1-3,13,14 Brox et al. reported nine cases of PSL, and splenomegaly was noted in 44% of these patients.15 In our cases, LUQ pain or discomfort was the presenting symptom in two cases, and constitutional symptoms with multiple small cervical lymphadenopathies were present in the third case. The spleen was not palpable on physical examination in any of our three cases. Significant laboratory findings of PSL may include cytopenia, or elevated ESR or β2-microglobulin level.2,3,16,17 In this report, elevated ESR and β2-microglobulin levels were present in the first and third cases and cytopenia was present in the third case.
The most common appearance of PSL on diagnostic imaging studies is hypodense lesions on contrast-enhanced CT scans and hypoechoic lesions on sonography.13,18 The differential diagnosis of a solitary splenic mass should include benign entities such as haemangioma, lymphangioma, hamartoma, infarct, and abscess, as well as metastatic disease.13,18 PSL also has a clinically recognizable pattern. However, preoperative diagnosis of the condition has not been considered in over 50% of the cases in the literature.18,19 In our patients, splenic lesions were hypodense, and more clearly seen on the infused scans. Only one of the patients showed any intra-abdominal lymph node enlargement. Initial impressions of the CT scans included angiosarcoma, lymphoma, metastasis, pseudotumor, or hamartoma in these three cases. Dachman et al. suggested that the most common manifestation of PSL is a hypodense lesion on CT and hypoechoic lesions on sonography in symptomatic patients, those who do not have fever or a known primary tumor elsewhere. However, splenic biopsy or splenectomy may be required for definitive diagnosis.13 In our second unique case described here, the tumor invaded the adjacent organs, pancreas, and transverse colon. Karpeh et al. reported the first case of splenic lymphoma with pathologically documented neoplastic invasion to the colonic mucosa.16
If imaging studies strongly suggest the presence of PSL, needle aspiration biopsy (NAB) or core biopsy of the spleen may be substituted for splenectomy as a diagnostic tool. These techniques can reduce the mortality and morbidity resulting from splenectomy; however, they are not yet widely accepted.7,13
In 1966, Ahmann et al. grouped PSL into three stages depending on the extent of the disease determined either at surgery or by other studies in the immediate postoperative period.1 Stage I refers to those patients with tumor is limited to the spleen only. Stage II patients have involvement of the nodes in the splenic hilum, while stage III patients have involvement of the liver or lymph nodes beyond the splenic hilum. According to this staging system, the first case in our study was stage I, and the second and the third cases were stage III.
Histopathologically, all of the malignant lymphoma cell types can be encountered in PSL, and are related to prognosis. In our patients, all cell types were diffuse large B-cell lymphomas, the most common type of NHL in Korea,3 which were positive for immunohistochemical stain L26.1 Because PSL is a rare disease there are few reports about tumor histopathologic type and patient survival. In one study, Xiros et al. reported that 11 (38%) patients had low-grade and 18 (62%) had intermediate or high-grade NHL.14 Of these,20 of the 29 patients received splenectomies for diagnostic purposes, like in our cases, and only the lymph nodes of the splenic hilum were found to be involved in 4 patients.14
Treatment of PSL includes splenectomy, which also serves as a diagnostic and confirmatory modality.7,12,20 Although there is no clear consensus, splenectomy or radiation therapy without splenectomy might be considered if the diagnosis can be made without splenectomy.13 Morel et al. and Kim et al. suggested that splenectomy could be particularly useful in patients with prolonged survival, primarily in those patients whose cytopenia resolves after surgery and in those patients treated with adjuvant chemotherapy who are more tolerant to the chemotherapy.2,17 Reportedly, failure to correct thrombocytopenia after splenectomy was a poor prognostic factor.2,17 All the patients in this study underwent splenectomy. Splenectomy was also used as a confirmative tool to differentiate PSL from a splenic mass seen on imaging studies. In the third case, the thrombocytopenia corrected after splenectomy concomitantly with distal pancreatectomy and segmental resection of the transverse colon.
Unfortunately, no indications or suggestions as to what might be the best approach to patients with primary splenic presentation following splenectomy are available in the literature.7 Aside from splenectomy, the treatment modalities administered in our cases included local radiation therapy and chemotherapy. The first patient received CHOP chemotherapy only after splenectomy. The second patient received CHOP chemotherapy with local radiation after surgery due to local invasion of PSL, whereas the third patient was treated with ProMACE-CytaBOM chemotherapy only after splenectomy.
Presently, all three of our patients are still alive without evidence of relapse at follow-up periods ranging from 51 to 135 months. Although further studies are needed, our data suggest that chemotherapy, with or without radiotherapy, after splenectomy prolongs survival of PSL patients.
Ahman et al. reported that the 5-year survival rate of all his PSL patients was 31% and 40% for those with stage I and II. Survival of his patients with stage I or stage II PSL was considerably better than those with stage III.1 However, patients with Hodgkin's disease were also included in the analysis.9 In a later study by Kehoe et al., investigating the prognosis of 21 patients according to disease stage over a 5-year period, concluded that there was a significant difference between the prognosis for patients with stage I or II (43%) than those with stage III (14%).2,9 Brox et al. reported a median survival of 7.48 years in nine cases of PSL.15 In Xiros et al. documented that the median survival in groups undergoing diagnostic splenectomy was 24 months regardless of disease stage or chemotherapeutic regimen (mainly CHOP).14 In our report, all three cases have survived for durations ranging from 59 to 143 months, despite the differences in disease stage and adjuvant therapy. Our excellent survival rates, as compared to previous reports, may be attributed to adjuvant chemotherapy following splenectomy in all cases and, as for the locally advanced case, combining radiotherapy into the treatment regime.
The prognosis for intermediate grade lymphoma, after splenectomy and chemotherapy, is slightly worse than that for well-differentiated small-cell lymphoma.7
There is no consensus concerning the best treatment for PSL. Further study is warranted to compare the outcomes among the following modalities: splenectomy only, chemotherapy after splenectomy, radiation therapy after splenectomy, or radiation therapy with chemotherapy.
Further long-term observations of survival are needed from more cases before any conclusions can be drawn with respect to needle aspiration biopsy and core biopsy of the spleen without splenectomy.
Figures and Tables
 | Fig. 1Computed tomography (CT) scans of each case. A) Case 1- a huge splenic mass with homogenous high-density areas, including an area of low-density without perisplenic spread. B) Case 2- a well-localized and capsulated splenic mass with internal necrosis or hemorrhage and partial bulging of the pancreatic tail portion at the junctional lesion with spleen. C) Case 3- a heterogeneous, lobulated splenic mass measuring 10×8×8 cm with bulging contours, necrotic foci and focal calcification. |
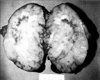 | Fig. 2Gross examination of the resected spleen of Case 1. The exposed cut surface shows a bulging, well-demarcated grayish-yellow nodular solid mass, measuring 12 cm in the largest dimension. Multifocal grayish-yellow necrotic foci are present. The splenic parenchyma is nearly replaced by this lesion. |
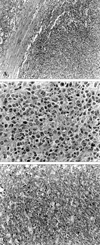 | Fig. 3Histopathologic findings of Case 1. A) Mass border on low power (×100) showing an expanding growth pattern of tumor cells. B) Mass on high power (×400) showing non-Hodgkin's lymphoma, diffuse large B-cell type. Tumor cells have large nuclei, open chromatin, and prominent nucleoli. C) Immunohistochemical stain for L26 (B-cell marker) (×200) showing prominent immunopositivity to the tumor cells. |
References
1. Ahmann DL, Kiely JM, Harrison EG, Payne WS. Malignant lymphoma of the spleen. Cancer. 1966. 19:461–469.
2. Kim KH, Cho CK, Choo SW, Kim HJ, Kim KS. Primary lymphoma of the spleen- a case report. Korean J Surg. 1997. 52:912–917.
3. Hahn JS, Lee S, Chong SY, Min YH, Ko YW. Eight-year experience of malignant lymphoma-survival and prognostic factors. Yonsei Med J. 1997. 38:270–284.
4. Fawcett DW. A textbook of histology. 1994. 12th ed. New York: Chapman & Hall;460–472.
5. Cotran RS, Kumar V, Robbins SL. Pathologic basis of disease. 1994. 5th ed. Philadelphia: W.B. Saunders;667–671.
6. McCormick WF, Kashgarian M. The weight of the adult human spleen. Am J Clin Pathol. 1965. 43:332–333.
7. Gobbi PG, Grignani GE, Pozzetti U, Bertoloni D, Pieresca C, Montagna G, et al. Primary splenic lymphoma: Does it exist? Haematologica. 1994. 79:286–293.
8. Kraemer BB, Osborne BM, Butler JJ. Primary splenic presentation of malignant lymphoma and related disorders-a study of 49 cases. Cancer. 1984. 54:1606–1619.
9. Kehoe J, Straus DJ. Primary lymphoma of the spleen-clinical features and outcome after splenectomy. Cancer. 1988. 62:1433–1438.
10. Falk S, Stutte HJ. Primary malignant lymphomas of the spleen. Cancer. 1990. 66:2612–2619.
11. Dasgupta T, Coombes BC, Brasfield RD. Primary malignant neoplasms of the spleen. Surg Gynecol Obstet. 1965. 120:947–960.
12. Skarin AT, Davey FR, Moloney WC. Lymphosarcoma of the spleen. Arch Intern Med. 1971. 127:259–265.
13. Dachman AH, Buck JL, Krishnan J, Aguilera NS, Buetow PC. Primary non-Hodgkin's splenic lymphoma. Clin Radiol. 1998. 53:137–142.
14. Xiros N, Economopoulos T, Christodoulidis C, Dervenoulas J, Papageorgiou E, Mellou S, et al. Splenectomy in patients with malignant non-Hodgkin's lymphoma. Eur J Haematol. 2000. 64:145–150.
15. Brox A, Bishinsky JI, Berry G. Primary non-Hodgkin lymphoma of the spleen. Am J Hematol. 1991. 38:95–100.
16. Karpeh MS Jr, Hicks DG, Torosian MH. Colon invasion by primary splenic lymphoma: a case report and review of the literature. Surgery. 1992. 111:224–227.
17. Morel P, Dupriez B, Gosselin B, Fenaux P, Estienne MH, Facon T, et al. Role of early splenectomy in malignant lymphomas with prominent splenic involvement (Primary lymphomas of the spleen)- A study of 59 cases. Cancer. 1993. 71:207–215.
18. Lee JD, Park CH, Griffith J, Vernick J. Gallium-67 scintiscan in the diagnosis of primary splenic non-Hodgkin's lymphoma after the treatment of Hodgkin's disease. J Nucl Med. 1992. 33:1183–1185.
19. Harris NL, Aisengerg AC, Meyer JE, Ellman L, Elman A. Diffuse large cell (histiocytic) lymphoma of the spleen. Cancer. 1984. 54:2460–2467.
20. Abraksia S, Dileep KP, Kasal J. Two unusual lymphomas. J Clin Oncol. 2000. 18:3731–3733.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download