Abstract
Pterygium is a proliferative disease. Recent research has reported that stem cells are involved in the pathogenesis of various proliferative diseases, including solid tumors and diabetic proliferate vitreoretinopathy. In previous literature, we hypothesized that adult stem cells originated from bone marrow were involved in the pathogenesis of pterygium. We proved this by immunohistochemical staining with various stem cell markers. The staining showed adult stem cells in the pterygium. c-kit positive cells were observed primarily in the stroma, and some cells were also found in the basal epithelium. AC133 and CD34 positive cells were primarily found in the basal epithelium and were ovoid shaped, similar to the c-kit cells. However, some cells were found in vascular endothelium. STRO-1 positive cells were found mainly in the stroma and were spindle shaped. In recurrent pterygium, cells were more scattered and the expression pattern was denser. Therefore, we suggest a new theory of pterygium pathogenesis. Inflammation caused by environmental factors triggers the abnormal production of some growth factors and cytokines in order to recover from cellular damage. If these healing signals are excessive, limbal basal cells will be changed to abnormally-altered pterygial cells. The excessive wound healing process and remnant altered cells result in recurrence using the same mechanism.
Pterygium is a chronic condition characterized by the invasion of altered ocular surface tissue into the cornea.1,2 The invasive cells show a transformed epithelium and fibroblasts in the conjunctiva.3,4 Ultraviolet light (UV), dust, etc. are known to be influential factors that induce this abnormal change of cells in pterygium pathogenesis.5 Until now, two opposite concepts have been proposed to explain pterygium pathogenesis. Pterygia have some characteristics of a degenerative disorder, such as elastoid and hyaline dystrophy, or thickening of basement membrane.6 However, proliferative features, such as epithelial hyperplasia, dys-generation of cell cycle related genes (high expression rate of mutated 53 and bcl-2 oncogene), and s high recurrence rate after excision, are also seen.7-9 Recent research results suggest that pterygium is a proliferative disease.10,11 Although this condition is well-studied, no studies to date have clearly explained its pathogenesis regarding the origin of cells and the mechanism of its aggressive recurrence behavior after surgical excision and the wound healing process.
Adult stem cells have several unique features. The cells originate from differentiated tissues, including bone marrow, and are capable of self-renewal. Thus, they can reproduce and proliferate on their own in adequate conditions.12-14 Recently, it was discovered that stem cells are involved in the pathogenesis of several proliferative diseases, including hematologic neoplasm,15 other solid tumors,16,17 paroxysmal nocturnal hemoglobinuria,18,19 and proliferate vitreoretinopathy.20
Based on these results, we hypothesized that certain environmental stimuli may stimulate multipotent bone marrow stem cells to gain the unexpected ability to proliferate and differentiate into phenotypically and functionally unrelated cells to the origin, then express various growth factors and cytokines, affecting proliferation, tissue invasion, and even recurrence of pterygium. Therefore, we performed immunohistochemical staining with various stem cell markers in pterygial tissues to investigate the involvement of the bone marrow-originated stem cells in the pathogenesis, progress, and recurrence of pterygium.
All procedures were performed under the tenets of the Helsinki declaration, and informed consent was obtained from all patients. Twenty consecutive pterygial tissues were obtained after surgical removal of the pterygium (primary pterygium, n = 10; recurrent pterygium, n = 10; mean age 55.1 and 56.4, respectively). Normal conjunctival tissues for the control group (n = 2) were obtained from healthy donors without any ocular surface disorders, such as allergies or dry eye. Paraffin embedded samples were used for AC133, c-Kit, and CD34. Snap-frozen sectioned tissues were used for STRO-1. Serial cross sections were made along the longitudinal axis so as to include the leading edge.
The avidin biotin complex technique was used to stain the sectioned specimens (2-4 µm). Paraffin sections were deparaffinized in xylene, rehydrated through decreasing ethanol grades, and quenched for endogenous peroxidase. Cryostat sections were placed on gelatinized slides, fixed in cold acetone, and then rinsed in tris-buffered saline (TBS). Nonspecific background was eliminated by incubating tissue sections with non-immuno serum (Zymed Laboratories, South San Francisco, CA, USA, Histostatin-plus Kits, Reagent A). Sections were incubated with monoclonal mouse anti-human AC133 antibody (Clone AC133 epitope, Miltenyi Biotech, Bergisch Gadbach, Germany), monoclonal mouse anti-human STRO-1 (DHBC, IA, USA), polyclonal goat anti-human CD34 (N-19, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and polyclonal goat anti-human c-Kit (C-14, Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4℃, then extensively washed in 0.05 M TBS (pH 7.6) before the addition of a biotinylated secondary antibody (Reagent B). Sections were washed again, incubated for 1 hour with peroxidase-conjugated streptavidin (Reagent C), and the presence of peroxidase was revealed by adding substrate-chromogen (3-amino-9-ethycarbazole) solution (Reagent D). Sections were counterstained with hematoxylin. Each section was photographed, and the entire tissue area was examined.
Upon light microscopic observation, abundant progenitor cells originated from bone marrow were seen in the basal epithelium or stroma of all primary (n = 10) and recurrent (n = 10) pterygial tissues. Positive immunoreactivity to progenitor cell markers were mainly found along the entire specimen axis, but were predominantly found in the head portion of the pterygium.
CD34 positive cells were observed in the basal epithelium of the pterygium, and cell shapes were ovoid to round. The staining results of CD34 were similar to those of c-kit in terms of the cell localization and expression pattern, but CD34 positive cells were found around the perivascular endothelium. Cell numbers and staining patterns were more prominent in recurrent pterygia, compared with the primary cases (Fig. 1. A-C).
c-Kit positive cells were observed not only in the stroma but were also found in the basal epithelium of the pterygium. Cell shapes were different from their stained sites. Cells observed in the stroma showed fibroblast-like and spindle-shaped morphology, whereas cells in the basal epithelial layer were round to ovoid-shaped. c-kit positive cells were not found in the normal conjunctiva (Fig. 2A).
AC133 positive cells were observed mainly in the epithelial layer, and some cells were scattered in the stroma of the pterygium. Most cells were ovoid-shaped. However, a few cells were spindle-shaped and these cells were not found in the normal conjunctiva. The sites of AC133 positive cells were similar to c-kit, and cells showed the same ovoid shape as CD34. Although the cell shapes and cell numbers varied, AC133, CD34, and c-kit positive cells were co-expressed in basal epithelium (Fig. 2B).
Spindle-shaped STRO-1 positive cells were found in the stroma by rhodanine staining (Fig. 2C). Generally, in recurrent pterygia positively stained sites and cell shapes were similar to those of the primary cases. However, cell numbers and expression patterns were more prominent in all recurrent cases compared with primary pterygia cases.
Adult stem cells originated from bone marrow play an important role in the pathogenesis and progression of various proliferative diseases.15-20 This role may be direct, through its proliferation or indirect, through the production of growth factors and cytokines. This may then induce adjacent cells to undergo unexpected propagation and vegetation properties without limitation.
Pterygium is one of the major sight-threatening conditions in developing countries. This condition creates several problems, including dry eye, irregular astigmatism, and unacceptable cosmetic trouble.1,2 Although some degenerative changes were observed,6 pterygium is considered a proliferative disorder.10,11 Many research results support this proliferative classification. Various growth factors and cytokines are expressed in the pterygia. Similar expressions are also found in some viral infections, such as those caused by the human papilloma virus11,21 and herpes simplex virus.22 These viral infections evoke gene mutation in the pterygium.
As a result of immunohistochemical staining in primary and recurrent pterygial tissue, various mesenchymal, hematopoietic, and endothelial progenitor cells were observed. c-Kit is identified on the cellular surface and it represents receptors of stem cell factor (SCF), also termed kit ligand, and it augments the proliferation of primitive bone marrow and mast cells. Positive staining for c-Kit indicates a mesenchymal lineage progenitor. c-Kit positive cells, found in the stroma and basal epithelium, had a spindle-shaped, fibroblast-like appearance. This suggested mesenchymal progenitor cells. CD34 is known as the most common marker for hematopoietic progenitor cells.23 CD34 positive cells were expressed in the basal epithelium and the peri-vascular endothelium. CD34 positive cells are hematopoietic precursors. Though there is no direct relationship between blood cells and pterygia, these hematopoietic precursors have a supportive role in other progenitor cells, such as mesenchymal lineages. AC133 is a marker for endothelial progenitors and neuronal stem cells. It is rapidly down-regulated during differentiation.24 AC133 positive cells were observed in similar sites as CD34, and with the same shape of round to ovoid. Based on the patterns of the AC133 and CD34 positive cells, we can suspect that these cells may be differentiated into the endothelium. It is strongly supports the above thought that AC133 and CD34 positive cells were found mainly in the basal epithelium and endothelium of small vessels of the stroma. STRO-1 is known as a potent marker for mesenchymal precursors.25,26 Positively stained cells are fibroblast-like and spindle-shaped. The staining site was overlapped in basal epithelium. This suggests that one type of stem cells was activated by another type of stem cells. Between each type of stem cell, orchestrated mechanisms occurred for migration and proliferation. As a result of stem cell markers and cell morphology staining, we can conclude that these cells were mesenchymal precursors and would be differentiated into fibroblasts, major transformed cells of the pterygium. The fact that both endothelial and mesenchymal lineages were involved in the progression of pterygium explains the rich fibrovascular bundles that encroached onto the cornea.
Generally, positive cells were found along the entire specimen axis, but predominantly in the head portion, which is known to be a very active proliferative area in a pterygium. In recurrent pterygia, they were more strongly expressed and increased in stem cells - a more scattered expression pattern and an increase in cell numbers. Stem cells were not found in control conjunctival tissue (data not shown). These results also provided strong evidence of stem cell involvement in pterygium pathogenesis, especially dys-regulated, uncontrolled proliferation of affected cells.
From our results and previously reported research, we propose a new theory for the pathogenesis and progression of pterygia. Stem cells are involved in pterygial pathogenesis, and recurrence after surgical removal is caused by the induction of abnormal differentiation and overproliferation of stem cells. Environmental factors known to be associated with pterygium pathogenesis, such as UV or dust, evoke inflammation and cell damage.5 These processes trigger the overproduction of growth factors and cytokines, such as TGF-β, IL-1β, IL-6, bFGF, IGFs, SCF, and MMPs,27-29 and conclusively enhance tissue remodeling. In this special ocular microenvironment, limbal basal cells were changed to abnormal pterygial cells and helped the encroachment of these altered pterygial cells into the cornea. During these processes, an unknown signal was transmitted to the bone marrow in order to enhance the migration of stem cells into the damaged ocular surface to repair damaged lesions via a rich vascular arcade of limbus. As a result, the healing process was activated and damaged surface was recovered. However, the excessive healing process led to the uncontrolled production of growth factors and cytokines. Cellular alteration into transformed epithelial cells and fibroblasts was observed in the pterygia, and finally, the disease developed. Our results support these conclusions. Moreover, from this hypothesis, one can approximate the presence of not only promoting factors, but also inhibitory factors for stem cells. Stem cells act as a double edged-sword in the healing process of a damaged ocular surface - recover or disorder. Our findings also explain the recurrent features of the pterygium after removal. Surgical excision causes more iatrogenic damages than environmental hazards on the ocular surface, and abundant growth factors and cytokines are produced. If abnormal microenvironments still affect the wound healing process, those products enhance cellular alteration, and transformed cells may infiltrate the ocular surface. Moreover, incomplete removal of altered cells promotes these processes, and recurrence is further accelerated. Therefore, stem cells may be an indicator for pterygial recurrence. It is reasonable to believe that larger numbers stem cells cause a higher recurrence rate of pterygia after removal. The complete removal of altered cells is essential to prevent recurrence of pterygium.
In this study, we proved that bone marrow originated stem cells are involved in pterygium pathogenesis, progression, and recurrence. This is a new idea and an important finding for pterygium pathophysiology. However, we revealed only a small portion of information related to pterygial pathogenesis, and pterygium still remains to be an ophthalmic enigma. Additional research studying factors that enhance or inhibit stem cells and triggering signals to bone marrow must be undertaken to further elucidate pterygial pathogenesis and to develop effective treatment methods without recurrence.
Figures and Tables
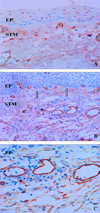 | Fig. 1Immunohistochemical staining of adult stem cells. CD34 positive cells were observed mainly in the basal epithelial portion of the pterygium (arrow). Cells were found in the vascular endothelium in the stroma (asterisk). Cell shapes were round to ovoid. In normal conjunctiva, a few positive cells were found. Perivascular endothelial cells were also positively stained (cross). (A: normal conjunctiva; B, C: pterygium). (EP, epithelium; STM, stroma) (A, B: × 200; C: × 400). |
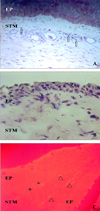 | Fig. 2Immunohistochemical staining of adult stem cells. c-Kit positive cells were observed mainly in the stroma and had a fibroblast like, spindle-shape (A: arrow). AC133 positive cells were found in the basal epithelium and stroma (B). STRO-1 positive cells were observed mainly in the stroma and had a fibroblast-like, spindle shape (C: asterisk). Cells in the basal epithelial layer of the pterygium (C: arrow head, rhodanine staining) (EP, epithelium; STM, stroma, × 200). |
References
1. Holland EJ, Mannis MJ. Ocular surface disease. Medical and surgical management. 2002. New York: Springer-Verlag;65–89.
2. Jaros PA, DeLuise VP. Pingueculae and pterygia. Surv Ophthalmol. 1988. 3:41–49.
3. Dushku N, John MK, Schultz GS, Reid TW. Pterygia Pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol. 2001. 119:695–706.
4. Maini R, Collison DJ, Maidment JM, Davies PD, Wormstone IM. Pterygial derived fibroblasts express functionally active histamine and epidermal growth factor receptors. Exp Eye Res. 2002. 74:237–244.
5. Saw SM, Tan D. Pterygium: prevalence, demography and risk factors. Ophthalmic Epidemiol. 1999. 6:219–228.
6. Austin P, Jakobiec FA, Iwamoto T. Elastodysplasia and elastodystrophy as the pathologic bases of ocular pterygia and pinguecula. Ophthalmology. 1983. 90:96–109.
7. Tan DT, Tang WY, Liu YP, Goh HS, Smith DR. Apoptosis and apoptosis related gene expression in normal conjunctiva and pterygium. Br J Ophthalmol. 2000. 84:212–216.
8. Chowers I, Pe'er J, Zamir E, Livni N, Ilsar M, Frucht-Pery J. Proliferative activity and p53 expression in primary and recurrent pterygia. Ophthalmology. 2001. 108:985–988.
9. Tan DT, Lim AS, Goh HS, Smith DR. Abnormal expression of the p53 tumor suppressor gene in the conjunctiva of patients with pterygium. Am J Ophthalmol. 1997. 123:404–405.
10. Weinstein O, Rosenthal G, Zirkin H, Monos T, Lifshitz T, Argov S. Overexpression of p53 tumor suppressor gene in pterygia. Eye. 2002. 16:619–621.
11. Varinli S, Varinli I, Koksal Erkisi M, Doran F. Human papillomavirus in pterygium. Cent Afr J Med. 1994. 40:24–26.
12. Dick JE. Stem cells: Self-renewal writ in blood. Nature. 2003. 423:231–233.
13. Horwitz EM. Stem cell plasticity: a new image of the bone marrow stem cell. Curr Opin Pediatr. 2003. 15:32–37.
14. Ohgushi H, Kitamura S, Kotobuki N, Hirose M, Machida H, Muraki K, et al. Clinical application of marrw mesenchymal stem cells for hard tissue repair. Yonsei Med J. 2004. 45:Suppl. 61–67.
15. Nowicki MO, Pawlowski P, Fischer T, Hess G, Pawlowski T, Skorski T. Chronic myelogenous leukemia molecular signature. Oncogene. 2003. 22:3952–3963.
16. Pathak S. Organ- and tissue-specific stem cells and carcinogenesis. Anticancer Res. 2002. 22:1353–1356.
17. Tu SM, Lin SH, Logothetis CJ. Stem-cell origin of metastasis and heterogeneity in solid tumours. Lancet Oncol. 2002. 3:508–513.
18. Risitano AM, Holada K, Chen G, Simak J, Vostal JG, Young NS, et al. CD34+ cells from paroxysmal nocturnal hemoglobinuria (PNH) patients are deficient in surface expression of cellular prion protein (PrPc). Exp Hematol. 2003. 31:65–72.
19. Nakakuma H, Kawakuchi T. Pathogenesis of selective expansion of PNH clones. Int J Hematol. 2003. 77:121–124.
20. Walshe R, Esser P, Wiedemann P, Heimann K. Proliferative retinal diseases: myofibroblasts cause chronic vitreoretinal traction. Br J Ophthalmol. 1992. 76:550–552.
21. Piras F, Moore PS, Ugalde J, Perra MT, Scarpa A, Sirigu P. Detection of human papillomavirus DNA in pterygia from different geographical regions. Br J Ophthalmol. 2003. 87:864–866.
22. Detorakis ET, Sourvinos G, Spandidos DA. Detection of herpes simplex virus and human papilloma virus in ophthalmic pterygium. Cornea. 2001. 20:164–167.
23. Krause DS, Ito T, Fackler MJ, Smith OM, Collector MI, Sharkis SJ, et al. Characterization of murine CD34, a marker for hematopoietic progenitor and stem cells. Blood. 1994. 84:691–701.
24. Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000. 95:952–958.
25. Gronthos S, Graves SE, Ohta S, Simmons PJ. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994. 84:4164–4173.
26. Dennis JE, Carbillet JP, Caplan AI, Charbord P. The STRO-1+ marrow cell population is multipotential. Cells Tissues Organs. 2002. 170:73–82.
27. Lee SB, Li DQ, Tan DT, Meller DC, Tseng SC. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000. 20:325–334.
28. Di Girolamo N, Kumar RK, Coroneo MT, Wakefield D. UVB-mediated induction of interleukin-6 and -8 in pterygia and cultured human pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2002. 43:3430–3437.
29. Li DQ, Lee SB, Gunja-Smith Z, Liu Y, Solomon A, Meller D, et al. Overexpression of collagenase (MMP-1) and stromelysin (MMP-3) by pterygium head fibroblasts. Arch Ophthalmol. 2001. 119:71–80.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download