Abstract
Spasticity has been defined as a motor disorder characterized by a velocity-dependent increase in tonic stretch reflex (muscle tone). Muscle tone consists of mechanical-elastic characteristics, reflex muscle contraction and other elements. The aims of this study were to determine whether to assess spasticity quantitatively, and to characterize biomechanical and electromyographic spasticity assessment parameters. These assessment parameters were described by investigating the correlation between clinical measures and the response to passive sinusoidal movement with consecutive velocity increments. Twenty post-stroke hemiplegic patients and twenty normal healthy volunteers were included in the study. Five consecutive sinusoidal passive movements of the ankle were performed at specific velocities (60, 120, 180, and 240 degrees/sec). We recorded the peak torque, work, and threshold angle using a computerized isokinetic dynamometer, and simultaneously measured the rectified integrated electromyographic activity. We compared these parameters both between groups and between different velocities. The peak torque, threshold angle, work, and rectified integrated electromyographic activity were significantly higher in the post-stroke spastic group at all angular velocities than in the normal control group. The threshold angle and integrated electromyographic activity increased significantly and linearly as angular velocity increased, but the peak torque and work were not increased in the post-stroke spastic group. Peak torque, work, and threshold angle were significantly correlated to the Modified Ashworth scale, but the integrated electromyographic activity was not. The biomechanical and electromyographic approach may be useful to quantitatively assess spasticity. However, it may also be very important to consider the different characteristics of each biomechanical parameter.
Spasticity, a common problem in upper motor neuron lesions, is classically defined as a motor disorder characterized by a velocity-dependent increase in tonic stretch reflexes (muscle tone) with exaggerated tendon jerks, which result from the hyperexcitability of the stretch reflex.1 Muscle tone may be characterized as "the sensation of resistance felt as one manipulates a joint through a range of motions, with the subject attempting to relax." Although this definition is adequate for the bedside examination, a more vigorous analysis indicated that muscle tone is likely to consist of several distinct components: (1) the physical inertia of the extremity, (2) the mechanical-elastic characteristics of the muscular and connective tissues, and (3) reflex muscle contraction (tonic stretch reflex).2,3
The ability to quantify spasticity is essential to the management of spasticity. For the quantification of spasticity, neurophysiologic approaches such as tendon jerk, H-reflex, tonic vibration reflex, electromyographic activity,4-8 and biomechanical approaches such as pendulum test and isokinetic dynamometer have been used.9-11
Although the modified Ashworth scale is commonly used to assess the severity of spasticity in the clinical setting, it is highly dependent on the examiner's judgment, its reliability is extremely low,12 and it is not valid at lower grades.13 Despite these limitations, it has been widely used in the study of spasticity. The tendon jerk, H-reflex, and tonic vibration reflex were poorly correlated with the clinical status of spasticity.5,14 The pendulum test is more quantitative than clinical measures, but most studies have focused on the knee joint.9-11
To overcome the limitations of the previous test, and to quantify spasticity more objectively, testing by an isokinetic dynamometer has been tried.15-17 An isokinetic dynamometer tests spasticity by measuring the resistance of a joint incurred by sinusoidal oscillation of the joint at different constant angular velocities, according to the definition by Lance et al.1 This isokinetic dynamometer system enables the investigator to standardize both velocity and angle of motion, and to objectively record the amount of force generated by the subject's muscles. The operation and interpretation is simple, and the procedure can be applied to a variety of joints and muscles. Other attempts to quantify spasticity used electromyography (EMG), which can capture the muscular reflex activity against stretching.4,7 Electromyographic assessment has a theoretical background which represents a more velocity-dependent reflex component than other assessment tools.15
Combinations of various biomechanical tests with simultaneous electromyographic recording have been studied.15,16,18-20 However, there have been few articles comparing the different typical characteristics and investigating the correlation between clinical measures and simultaneous neurophysiologic or biomechanical tests.
In this study, we tried to quantify the spasticity of ankle plantar flexors in post-stroke spastic hemiplegic patients by simultaneous isokinetic dynamometer and integrated EMG activity assessment. The aims of this study were to determine whether to assess spasticity quantitatively, and to characterize the biomechanical and electromyographic spasticity assessment parameters by investigating the clinical correlation with the modified Ashworth scale and the response to passive sinusoidal movement with consecutive velocity increments.
Twenty post-stroke adult patients and twenty normal healthy adults were included in this study. The post-stroke spastic group included 16 males and 4 females. Their age was 51.1 ± 12.6 years, and the duration from onset was 5.8 ± 1.9 months. Twelve cases had an infarction in the middle cerebral artery territory, and eight cases had intracerebral hemorrhages in the basal ganglia. Eleven cases were right hemiplegic patients, and nine were left hemiplegic patients. All of the post-stroke patients had the increased ankle jerk and various degrees of ankle clonus on the hemiplegic side. We excluded the patients that had a history of musculoskeletal or neurological disorders of the ankle joint, limited range of motion of ankle joint, severe spasticity (such as modified Ashworth scale Grade 4), or had such poor cognitive function that they could not obey the examiner's orders.
The normal control group included 14 males and 6 females who had no musculoskeletal or neurological disorders. Their age was 46.9 ±13.9 years. The examined sides were divided equally between right and left, with 10 for each side. We didn't find any significant differences in sex or age between the post-stroke spastic group and the normal control group.
The modified Ashworth scale of each subject's ankle plantar flexor was evaluated by experienced physiatrists who were uninformed of the biomechanical assessment results.21 The examiners placed one hand under the ball of the foot, while the other hand stabilized the limb around the ankle joint. The subject's ankle was then moved passively and quickly into dorsiflexion. The resistance was then scored into the modified Ashworth scale. The subjects were asked to relax during the procedure.
Immediately after the modified Ashworth scale measurement, the subjects were positioned prone, with the knee straight, on the padded chair of an isokinetic dynamometer (Cybex 6000, Lumex Inc, Ronkonkoma, NY, USA). This system allowed for independent control of angular velocity acceleration, angular displacement, and positional error threshold. The footplate and binding were adjusted to ensure a snug fit and the optimal alignment of the ankle's axis of rotation with the footplate shaft. All subjects were instructed to relax. The passive dorsiflexion and plantar flexion movements were imposed on the ankle with the angular velocity of 60, 120, 180, and 240 degrees/sec, in that order. The experimental setting is shown in Fig. 1. The torque was measured by the resistance forces applied to the lever during the passive motion of the ankle joint from 20° plantar flexion to 10° dorsiflexion, as this range was common to all of the subjects tested, and is likely to approximate the range of motion achieved during gait. The parameters measured were peak torque (Nm), threshold angle (°), and work (Joule). The peak torque was defined as the peak resistance force during the passive movement. The threshold angle was defined as the angle at which torque rises from the zero torque line. An increased threshold angle meant that the torque was generated earlier, because this threshold angle was the plantar flexion angle of the ankle joint. Work was defined as the sum of torques at each angular velocity, and was calculated from the areas of the torque angle curve; the boundaries for the area were the torque-angle curve and the zero torque line. Analysis was done using five repetitions at each speed.
At the same time, we recorded the electromyographic activity obtained from the gastrocnemius. The active electromyographic electrode was placed lateral the muscle belly, and the reference electrode was placed along the midline of the posterior leg, just distal to the gastrocnemius insertion into the Achilles tendon. The raw electromyographic activity, which was produced by the stretch reflex related to the passive movement of ankle joint, was recorded with Ag-AgCl surface electrodes, and it was fully rectified and amplified with a bandpass filter (10-5000 Hz). The electromyographic activity was integrated using a 16-bit A/D converter (BIOPAC Inc, Goleta, CA, USA). Through the above procedure, we recorded the integrated electromyographic activity for the passive movement at each velocity (amplitude units/sampling rate). Also, we applied an electro-goniometer to present the ankle movement as a sinusoidal curve on the monitor. Thus, we were able to detect the exact point of departure from the baseline. In addition, we considered this departure point as the bursting point of electromyographic activity.
We compared these parameters between the post-stroke spastic group and the normal control group at each passive movement velocity using independent Student's t-tests. We evaluated the changes in these parameters during the angular velocity increment for the post-stroke spastic group and normal control group using repeated ANOVA. Also, we investigated whether the parameters had a linear relationship with the passive movement velocity using linear regression analysis. We calculated Pearson's correlation coefficients to analyze the relationship between the modified Ashworth scale and all parameters. To analyze this, the modified Ashworth scale was scored by setting Grade 1 to 1, Grade 1+ to 2, Grade 2 to 3, Grade 3 to 4, and Grade 4 to 5, as Skold et al.8 had done. Statistical significance was defined as p < 0.05.
All parameters, including peak torque, work, threshold angle, and integrated electromyographic activity, were significantly higher in the post-stroke spastic group at all angular velocities than in the normal control group (Table 1).
Repeated ANOVA revealed a significant interaction between Angular Velocity 60, 120, 180, 240 × Group normal, spastic on peak torque (F=11.756; p < 0.01), indicating a significant difference in changes of peak torque during the angular velocity increments between groups (Fig. 2). The peak torque tended to decrease from 60 to 180 degrees/sec as angular velocity increased in both groups, but the significant decrease appeared only from 60 to 120 degrees/sec of angular velocity (Table 1; Fig. 2). Interestingly, the peak torque at 240 /sec increased significantly in the normal control group, compared to that at 180°/sec angular velocity (Table 1). Regression analysis demonstrated the significant linear decrease in peak torque values associated with increasing velocity in the post-stroke spastic group, but the normal control group did not show a linear correlation after regression analysis (Table 2, Fig. 2, p < 0.05).
In terms of work, repeated ANOVA revealed a strong trend in the interaction of Angular Velocity 60, 120, 180, 240×Group normal, spastic and work (F=2.416; p=0.07), indicating a different trend in changes in work during the angular velocity increment between groups (Fig. 3). The work significantly increased as angular velocity increased for each respective angular velocity in the normal control group (p < 0.05). However, in the post-stroke spastic patient group, we didn't find any significant changes as angular velocity increased (Table 1, Fig. 3). Regression analysis demonstrated that the work increased linearly associated with increasing velocity in the normal group (p < 0.05), but the post-stroke spastic group did not show any linear correlations after regression analysis (Table 2, Fig. 3).
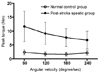
Repeated ANOVA revealed a significant effect of Angular Velocity 60, 120, 180, 240×Group normal, spastic on threshold angle (F=21.006; p < 0.01) and integrated EMG activity (F=9.848; p < 0.01), indicating a significant difference in changes of threshold angle and integrated EMG activity during the angular velocity increments between groups (Fig. 4 and 5). The threshold angle and the integrated electromyographic activity increased significantly as angular velocity increased for each respective angular velocity in both groups (Table 1, Fig. 4 and 5, p < 0.05). Regression analysis demonstrated that there were significant linear increases in threshold angle and integrated electromyographic activity associated with increasing velocity in both groups (Table 2, Fig. 4 and 5, p < 0.05).
Of the twenty post-stroke patients, the modified Ashworth scale for grading spasticity was grade 2 in seven patients, grade 1+ in seven patients, grade 1 in four patients, and grade 3 in one patient. The modified Ashworth Scale ranged from 1 to 3.
The peak torque, work, and threshold angle, all parameters of isokinetic assessment, were significantly correlated with the modified Ashworth scale for clinical spasticity (p < 0.05), but the integrated electromyographic activity was not correlated with the scale. Table 3 shows the correlation coefficients between the biomechanical assessment parameters and the modified Ashworth scale.
Spasticity frequently results in uncontrolled involuntary motion that interferes with function. When the spasticity is combined with other motor deficits in stroke patients, it may substantially increase motor and balance control impairment. Also, uncontrolled and sustained spasticity may lead to secondary disabling contractures, e.g., equinus deformity of the foot. Therefore, the proper assessment and management of spasticity is very important in the rehabilitative treatment of stroke patients.5,6,17,22
For the quantification of spasticity, biomechanical measurements have been used for the last decade, and the isokinetic dynamometer and electromyographic assessment have been used frequently. As parameters of isokinetic dynamometer measurement, peak torque, threshold angle, and work were used in previous studies.4,14-17,23-26 Many authors tried to determine whether the parameters of the isokinetic dynamometer were appropriate for the quantification of spasticity. They also tried to correlate their biomechanical evaluation results with clinical evaluations of spasticity.4,13,18,26 However, the results of each study were different. In order to make meaningful quantitative measurements, it is essential to match the biomechanical and clinical aspects. In reality, the matches between the basis of a good definition and what happens in most biological systems are not as close as they might be.27 Therefore, we tried to characterize these parameters in the clinical and definition-compatible aspects.
The post-stroke spastic group showed higher values than the normal control group for all of the parameters from the isokinetic evaluation with electromyographic activity, at all angular velocities, in our study. Therefore, we thought that peak torque, work, threshold angle, and integrated electromyographic activity might be useful for the detection of the increased muscle tone. Many researchers reported similar results.4,14-17,23-26
However, we did not observe a significant linear increase in peak torque and work values associated with increasing velocity; in fact, the peak torque decreased as angular velocity increased. The linear relationship between the resistance torque and angular velocity in post-stroke spastic subjects has been debated. Some previous researchers reported similar results,28,29 but some researchers did not.5,6,24,30 Peak torque refers to the resistance of the ankle plantar flexor against the passive range of motion or stretching. This resistance can arise as a result of at least two specific changes: altered muscle function and/or altered mechanical properties of the muscle.15,17,19,20 Skold29 examined the spasticity of bilateral quadriceps and hamstring muscles of twenty spinal cord injury patients by isokinetic dynamometer. In his study, the patients had a passive movement session during the six-week period. He found that the immediate effect, evaluated by both a self-rating visual analogue scale and a clinical modified Ashworth scale measurement, showed a reduction of spasticity. However, he did not illustrate the reason for the acute decrease of the spasticity after passive movement. Nuyens et al.28 found that stroke patients presented a significantly stronger torque reduction during the passive repetitive movements at all speeds tested compared to controls, and that the effect increased with increasing speeds. He suspected that the passive movements of the knee induced a decrease in spastic hypertonia in stroke patients through a combination of reflexive and mechanical factors.
These two studies support our results that the peak torque, the resistance force to passive movement, might be affected by not only hyperactive phasic stretch reflexes, but also by altered mechanical properties and histopathologic finding of the ankle plantar flexor after paresis or immobilization. We thought that the lack of increase of the peak torque with increased angular velocity might be due to the passive stretching of ankle plantar flexor while isokinetic dynamometer repeated plantar flexion and dorsiflexion. Also, the mechanism of spasticity suppression can be caused by presynaptic inhibition through primary spindle afferent firing or neurotransmitter depletion. However, we didn't have any positive and supportive results. Our regression analysis demonstrated that there was a significant linear increase in the integrated electromyographic activity values associated with increasing velocity. Also, the hypertonus has active (reflex) and passive (non-reflex) components. These results may be caused by the changes in passive (non-reflex) components in the spastic muscle. Given et al.25 described the histologic characteristics of the ankle plantar flexor, which is rich in connective tissue and slow-twitch fibers and thus vulnerable to immobilization. Booth et al.31 reported that there was a highly significant correlation between the modified Ashworth Scale and collagen content in children with chronic cerebral palsy. Thus, we thought that peak torque and work might be mainly affected by the changes in mechanical characteristics in the ankle plantar flexor muscle. Kartz et al.26 suggested similar opinions that the torque measurements are heavily influenced by limb size and other viscoelastic properties (e.g., contracture) within the spastic limb.
In the normal control group, the peak torque at 240°/sec angular velocity was higher than it was at 180°/sec angular velocity. We didn't know the exact reason, but we suspect that this finding may be a defensive reaction against overly fast angular velocity.
The threshold angle increased as angular velocity increased significantly for each respective angular velocity. The threshold angle represents the muscular sensitivity of stretching, and may have a more reflexic component than the peak torque and work. Our results suggested that threshold angle might be one of the reflexic components. Rymer and Katz32 introduced two parameters, reflex threshold and reflex gain, that may be altered in the pathologic stretch reflex in spasticity. As a joint is extended, the torque elicited by muscle stretch begins to increase. They defined the reflex threshold as the joint angle at which torque was initially generated. The reflex gain was defined as the slope of the torque increase according to muscle stretch. They reported that reflex gain was not enhanced, and that reflex threshold was the principle abnormality in spastic hypertonia. Electromyographic activity has been used to check unwanted activity and the reflex response during high velocities in isokinetic measurements.15 The integrated electromyographic activity increased as angular velocity increased in our study. The result of integrated electromyographic activity was similar to the torque threshold angle. Thus, we thought that the threshold angle and integrated electromyographic activity were reflexic and have velocity-dependent characteristics, which is compatible with the definition of spasticity.
Comparing the clinical spasticity measure (modified Ashworth scale) to the isokinetic and integrated electromyographic assessment of spasticity, the modified Ashworth scale yielded a statistically significant correlation with all three parameters of the isokinetic assessment, but not with integrated electromyographic activity. Damiano et al.33 reported similar findings, in which the Ashworth scores were correlated with instrumented measures, with higher correlations to the rate of change in resistance (stiffness) and the onset angle of stretch than to peak resistance torque. Skold et al.20 reported that peak torque and maximum electromyographic activity, as well as actual electromyographic activity at peak torque, showed no significant correlations, and that the resistive muscle torque seemed to measure the passive viscoelastic components rather than the active spastic components of the movement-provoked muscle resistance. The electromyographic activity may be representative of the reflexic component of spasticity, but not that of the viscoelastic component, so it might evoke the result that the electromyographic activity was not correlated with the clinical score.
We concluded that the isokinetic and integrated electromyographic assessments may be useful to assess spasticity, based on the fact that those of the post-stroke spastic hemiplegic patients were significantly different from those of normal healthy persons at all angular velocities. The peak torque, torque threshold angle, and work correlated closely with the modified Ashworth scale. Although all three of these parameters can detect and quantify the muscular tone change, the threshold angle may be most precise for the quantification of hyperactive phasic stretch reflex according to various angular velocities. The integrated electromyographic assessment does not correlate with the modified Ashworth scale score, but, like the threshold angle, is a valuable tool for the quantification of hyperactive phasic stretch reflex according to various angular velocities. We think that the different characteristics between isokinetic and integrated electromyographic assessment should be considered in evaluating the effects of therapeutic intervention of spasticity and in quantifying spasticity.
Figures and Tables
Fig. 1
Experiment setting for biomechanical assessment with electromyography of ankle plantar flexor spasticity.
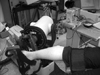
Fig. 5
Changes in integrated electromyographic activity with respect to angular velocity Increments.

Table 1
Peak Torque, Work, Threshold Angle and Integrated Electromyographic Activity at Different Angular Velocities

References
1. Lance JW. Feldman RG, Young RR, Koella WP, editors. Symposium Synopsis. Spasticity: Disordered motor control. 1980. 1st ed. Chicago: Year Book Publishers;485–494.
2. Pinelli P, Villani A, Pasetti C, Pisano F, DiLorenzo G. Delwaide PJ, Young RR, editors. Electromyographic and kinesiological evaluation of spastic hemiplegic hand. Clinical neurophysiology in spasticity: contribution to assessment and pathophysiology. 1985. 1st ed. Amsterdam: Elserver;141–154.
3. Wyke B. Neurological mechanisms in spasticity: a brief review of some current concepts. Physiotherapy. 1976. 62:316–319.
4. Allison SC, Abraham LD. Correlation of quantitative measures with the modified Ashworth scale in the assessment of plantar flexor spasticity in patients with traumatic brain injury. J Neurol. 1995. 242:699–706.
5. Benecke R, Conrad B, Meinck HM, Hohne J. Electromyographic analysis of bicycling on an ergometer for evaluation of spasticity of lower limbs in man. Adv Neurol. 1983. 39:1035–1046.
6. Firoozbakhsh KK, Kunkel CF, Scremin AM, Moneim MS. Isokinetic dynamometric technique for spasticity assessment. Am J Phys Med Rehabil. 1993. 72:379–385.
7. Lin JP, Brown JK, Brotherstone R. Assessment of spasticity in hemiplegic cerebral palsy. II: Distal lower-limb reflex excitability and function. Dev Med Child Neurol. 1994. 36:290–303.
8. Skold C, Harms-Ringdahl K, Hultling C, Levi R, Seiger A. Simultaneous Ashworth measurements and electromyographic recordings in tetraplegic patients. Arch Phys Med Rehabil. 1998. 79:959–965.
9. Bajd T, Vodovnik L. Pendulum testing of spasticity. J Biomed Eng. 1984. 6:9–16.
10. Brown RA, Lawson DA, Leslie GC, Part NJ. Observations on the applicability of the Wartenberg pendulum test to healthy, elderly subjects. J Neurol Neurosurg Psychiatry. 1988. 51:1171–1177.
11. Stillman B, McMeeken J. A video-based version of the pendulum test: technique and normal response. Arch Phys Med Rehabil. 1995. 76:166–176.
12. Sloan RL, Sinclair E, Thompson J, Taylor S, Pentland B. Inter-rater reliability of the modified Ashworth Scale for spasticity in hemiplegic patients. Int J Rehabil Res. 1992. 15:158–161.
13. Pandyan AD, Price CI, Barnes MP, Johnson GR. A biomechanical investigation into the validity of the modified Ashworth Scale as a measure of elbow spasticity. Clin Rehabil. 2003. 17:290–293.
14. Katz RT, Rymer WZ. Spastic hypertonia: mechanisms and measurement. Arch Phys Med Rehabil. 1989. 70:144–155.
15. Boiteau M, Malouin F, Richards CL. Use of a hand-held dynamometer and a Kin-Com dynamometer for evaluating spastic hypertonia in children: a reliability study. Phys Ther. 1995. 75:796–802.
16. Gottlieb GL, Agarwal GC, Penn R. Sinusoidal oscillation of the ankle as a means of evaluating the spastic patient. J Neurol Neurosurg Psychiatry. 1978. 41:32–39.
17. Lehmann JF, Price R, deLateur BJ, Hinderer S, Traynor C. Spasticity: quantitative measurements as a basis for assessing effectiveness of therapeutic intervention. Arch Phys Med Rehabil. 1989. 70:6–15.
18. Allison SC, Abraham LD, Petersen CL. Reliability of the Modified Ashworth Scale in the assessment of plantarflexor muscle spasticity in patients with traumatic brain injury. Int J Rehabil Res. 1996. 19:67–78.
19. O'Dwyer NJ, Ada L, Neilson PD. Spasticity and muscle contracture following stroke. Brain. 1996. 119:1737–1749.
20. Skold C, Harms-Ringdahl K, Seiger A. Movement-provoked muscle torque and EMG activity in long-standing motor complete spinal cord injured individuals. J Rehabil Med. 2002. 34:86–90.
21. Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987. 67:206–207.
22. Corcos DM, Gottlieb GL, Penn RD, Myklebust B, Agarwal GC. Movement deficits caused by hyperexcitable stretch reflexes in spastic humans. Brain. 1986. 109:1043–1058.
23. Akman MN, Bengi R, Karatas M, Kilinc S, Sozay S, Ozker R. Assessment of spasticity using isokinetic dynamometry in patients with spinal cord injury. Spinal Cord. 1999. 37:638–643.
24. Engsberg JR, Olree KS, Ross SA, Park TS. Quantitative clinical measure of spasticity in children with cerebral palsy. Arch Phys Med Rehabil. 1996. 77:594–599.
25. Given JD, Dewald JP, Rymer WZ. Joint dependent passive stiffness in paretic and contralateral limbs of spastic patients with hemiparetic stroke. J Neurol Neurosurg Psychiatry. 1995. 59:271–279.
26. Katz RT, Rovai GP, Brait C, Rymer WZ. Objective quantification of spastic hypertonia: correlation with clinical findings. Arch Phys Med Rehabil. 1992. 73:339–347.
27. Johnson GR. Outcome measures of spasticity. Eur J Neurol. 2002. 9:Suppl 1. 10–16. dicussion 53-61.
28. Nuyens GE, De Weerdt WJ, Spaepen AJ Jr, Kiekens C, Feys HM. Reduction of spastic hypertonia during repeated passive knee movements in stroke patients. Arch Phys Med Rehabil. 2002. 83:930–935.
29. Skold C. Spasticity in spinal cord injury: self- and clinically rated intrinsic fluctuations and intervention-induced changes. Arch Phys Med Rehabil. 2000. 81:144–149.
30. Singer BJ, Dunne JW, Singer KP, Allison GT. Velocity dependent passive plantarflexor resistive torque in patients with acquired brain injury. Clin Biomech (Bristol, Avon). 2003. 18:157–165.
31. Booth CM, Cortina-Borja MJ, Theologis TN. Collagen accumulation in muscles of children with cerebral palsy and correlation with severity of spasticity. Dev Med Child Neurol. 2001. 43:314–320.
32. Katz RT. Rymer WZ, Katz RT, editors. Physical Medicine and Rehabilitaton: state of the art reviews on spasticity. Mechanisms of spastic hypertonia. 1994. 8th eds. Philadelphia: Hanley & Belfus, Inc;441–454.
33. Damiano DL, Quinlivan JM, Owen BF, Payne P, Nelson KC, Abel MF. What does the Ashworth scale really measure and are instrumented measures more valid and precise? Dev Med Child Neurol. 2002. 44:112–118.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download