Abstract
Pancreatic cancer is a disease with poor prognosis mainly due to low resection rates and late diagnosis. To increase resectability and improve survival rates, a better understanding of pancreatic cancer pathogenesis and more effective screening techniques are required. New methods, such as genetic and molecular alterations, may suggest novel approaches for pancreatic cancer diagnosis and treatment. We immunohistochemically investigated 44 formalin-fixed, paraffin-embedded specimens of pancreatic ductal adenocarcinoma using monoclonal anti-p16 antibodies and monoclonal anti-p53 antibodies. The expressions of p16 and p53 proteins were compared using the Chi-square test with SPSS. Disease-free survival was analyzed using the Kaplan-Meier method, verified by the Log-Rank test. Loss of p16 expression was noted in 20 (45.5%) cases and aberrant p53 protein expression was detected in 14 (31.8%) cases. Loss of p16 expression was associated with a higher incidence of lymph node metastasis (p=0.040) and a more advanced stage (p=0.015), although there was no significant correlation between p16 expression and survival. Aberrant p53 protein expression correlated with histologic grade (p=0.038). Disease-free survival rate was significantly lower in the aberrant p53 protein positive group compared to the negative group (p=0.029). From our results, we suggest that p53 is not a prognostic factor; however, p16 and p53 genes do play important roles in the progression of pancreatic ductal adenocarcinoma.
According to statistics from the 1999 Ministry of Health & Welfare of South Korea, pancreatic cancer ranked 8th and 10th among cancers in incidence in men and women, respectively, and the rate is increasing.1 Smoking, high-calorie diets, certain chemical exposures, chronic pancreatitis, cystic fibrosis, and diabetes are known to induce pancreatic cancer; however, no exact cause has been identified.2
Even after curative resection, pancreatic cancer has a poor prognosis due to low resection rates and late diagnosis. Only 15-20% of cases undergo resection, and 5-year survival is approximately 20%.3,4 In addition, adjuvant therapies have not yet been standardized.
Several oncogenes and tumor suppressor genes have been shown to be associated with cancer pathogenesis, and molecular-based treatment approaches are being attempted for various cancers, including pancreatic cancer.5 Major genes related to pancreatic cancer include K-ras, p53, p16, and DPC4.6-8
The p16 gene, also called MST1 or CDKN2, is a tumor suppressor gene located on chromosome 9p21. By binding to cyclin-dependent kinase 4, the p16 protein inhibits phosphorylation of various growth and regulation factors which control proliferation in the G1 period of the cell cycle. When p16 genes are mutated, cyclin-dependent kinase 4 activation is increased and hyperphosphorylation of Rb protein occurs, leading to accentuated progression of the cell cycle from G1 to S and to cell proliferation.
P53 is located on chromosome 17p; it is a cell cycle checkpoint and induces apoptosis. If p53 gene functions are inactivated, control of cell proliferation and induction of cell apoptosis, two major regulations in cell growth, are lost.
Ongoing studies evaluating the association between genes and the clinical or biological properties of pancreatic cancer have shown various results. In addition, current studies aim to identify the carcinogenic phase, in which various tumor suppressor genes and oncogenes are converted from normal cells to cancer cells.
The aims of this study were to detect p16 protein expression loss and p53 protein overexpression in pancreatic cancer using immunohistochemical staining, to analyze the clinicopathologic characteristics and their relation to survival rate and to determine the clinical significance of p16 and p53 genes and their interaction.
Patients diagnosed with pancreatic ductal adenocarcinoma who underwent curative resection in the Department of Surgery, Yonsei University, College of Medicine between March 1990 and April 1999 were eligible for the study. Ultimately, 44 subjects with appropriate paraffin-embedded tissue samples and follow-up data were selected.
We retrospectively examined medical and pathological records to identify clinical and histopathological characteristics of subjects.
The 5 µm serial sections of each block were adhered to poly-L-lysine covered slides, and heated at 50℃ for 1 hour. After paraffin elimination with Xylene and a staged ethyl alcohol dehydration, sections were placed in methanol (300 ml) and hydrogen peroxide (10 mL) for 20 minutes in order to saturate endogenous peroxidase. After washing in water, sections were placed in citrate buffer solution and heated for 20 minutes. After cooling, samples were processed with TRIS buffer solution for 10 minutes. Sections were then incubated for 12 hours with p16 antibody (Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA), diluted at a 1:100 ratio. After washing with TRIS buffer solution, sections were stained using the Universal LSAB peroxidase II kit (Dako, Carpenteria, CA, USA) and developed by diaminobenzidine.
A 1:200 dilution ratio of p53 antibody (Novocastra, Benton, NC, UK) was used for p53 immunohistochemical staining. The remaining procedure was identical to the p16 protein immunohistochemical staining process.
Positivity for p16 and p53 immunohistochemical staining was microscopically determined by identification of dark brown-stained nuclei. The sample was considered p16 or p53 protein positive in cases that had 5% or greater positive cells from 1,000 cells under high magnifying power (×400).
Of the 44 subjects, there were 30 men and 14 women; the average age was 57 (range: 39 to 75) years. Thirty-eight tumors were at the head of the pancreas (86.4%) and 6 were in the body or tail (13.6%); more were located at the head, as tumors in that location have greater curative resection possibility than those in the body or tail.
Cell types included 41 (93.2%) cases of ductal adenocarcinoma and 3 (6.8%) cases of mucinous ductal adenocarcinoma. Of the ductal adenocarcinoma cases, 6 (14.6%) were well-differentiated, 21 (51.2%) were moderately-differentiated, and 14 (34.1%) were poorly-differentiated. Size and depth of tumor invasion classified by the American Joint Committee on Cancer (AJCC) criteria of T1, T2, T3, and T4 class were 3 (6.8%), 2 (4.5%), 35 (79.6%), and 4 (9.1%) cases, respectively; T3 was the most common class in our study population. Lymph node metastasis was evident in 19 (43.2%) cases. According to AJCC staging, the number of patients with stage I, II, III and IV cancer were 4 (9.1%), 18 (40.9%), 18 (40.9%), and 4 (9.1%), respectively (Table 1).
By immunohistochemical staining, there were 20 (45.5%) cases with negative p16 protein expression and 14 (31.8%) cases with positive p53 protein expression (Fig. 1, 2).
Negative p16 protein expression occurred in 13 (43.3%) of 30 men and 7 (50.0%) of 14 women, indicating no difference between the sexes. Negative p16 protein expression was not significantly different among histologic differentiation or T-staging. Negative expression occurred in 3 (50.0%) well-differentiated, 11 (52.4%) moderately-differentiated, and 5 (35.7%) poorly-differentiated tumors, and in 2 (75.0%), 1 (50.0%), 14 (40.0%), and 3 (75%) cases in T1, T2, T3, and T4 classes, respectively. There was a statistically significant difference of negative p16 protein expression between cases with and without lymphatic metastasis, 8 (32.0%) and 12 (63.2%), respectively (p=0.040). There was also a statistically significant difference between stage I and II vs. stage III and IV cases, 6 (27.3%) and 14 (63.6%), respectively (p=0.015) (Table 2).
Overexpression of p53 protein was demonstrated in 11 (36.7%) men and 3 (21.4%) women, showing no difference among sex. With regards to histologic differentiation, overexpression was present in 0 (0.0%), 7 (33.3%), and 7 (50.0%) cases with well-, moderately, and poorly differentiated tumors, respectively; thus indicating that frequency of p53 overexpression is correlated to extent of differentiation (p=0.038). There was no significant difference between expression of p53 protein and tumor size, depth of invasion, lymph node metastasis, or pathologic staging (Table 3).
The overall disease-free survival rate of patients with and without p16 protein expression was 18 and 21 months, respectively, showing no statistically significant difference (Fig. 3). However, disease-free survival of patients with and without p53 overexpression was 10 and 24 months, respectively (p=0.0209) (Fig. 4).
Pancreatic cancer is a deadly disease with no effective therapy besides surgical resection. Although there have been many studies, none have yet elucidated the cause of poor prognosis in pancreatic cancer. Due to developments in molecular biology, there has been rapid progress in the understanding of the genetic aspects of cancers. Identifying genetic changes in cancer contributes not only to detecting the cause of cancer, but also to developing novel methods for diagnosis and treatment.
The mutation of p16 genes in pancreatic cancer is caused by homozygous deletion, point mutation, or methylation of the CpG island located in the p16 gene promoter region.6,9,10 The incidence of p16 gene mutation and protein expression in pancreatic cancer has been reported between 30 and 87%,8,10-12 with the wide range possibly caused by a difference in subjects or examinations. Prior studies have showed that compared to other tissues, pancreatic cancer cell lines had more p16 gene mutation; results were different based on mutation study methods and immunohistochemical staining. The results of this study, in which p16 protein expression was lost in 45.5% of patients, were similar to those of previous studies.
A recent study reported that p16 genes play an important role in the carcinogenesis of pancreatic cancer, and Hu et al.11 stated that comparison of p16 protein expression in normal pancreatic tissue, acute pancreatitis, cystic tumor of the pancreas, and pancreatic cancer produced a significant difference. Moskaluk et al.13 observed that the incidence of p16 mutation is high in early intraductal pancreatic cancer lesions with K-ras mutations, and stated that such mutations contributed to the development of infiltrating cancer. Another study, by Wilentz et al.14 had similar results, and reported that p16 genes played an important role in carcinogenesis of pancreatic cancer.
Many different results have been published regarding p16 gene association with clinicopathologic properties and survival rate. Bartsch et al.15 stated that median survival of cases with and without p16 mutation was 8.5 and 17 months, respectively. However, other studies,9,12 including our study, found no relationship between p16 gene overexpression and survival rate. Our results showed that loss of p16 expression is associated with lymphatic metastasis and pathologic stage. There are other reports showing that loss of p16 expression is associated with extent of histologic differentiation. However, no reports have yet demonstrated the association with lymphatic metastasis or tumor stage. Because lymphatic metastasis plays a critical role in discriminating stage II from stage III, we suggest that the stages are related to loss of p16 protein expression. Further studies using tissue from lymphatic metastasis are necessary.
Most of the p53 gene mutation in pancreatic cancer is caused by point mutation or loss of heterozygosity.3 The half-life of normal p53 protein is short (10-20 minutes), but that of mutant p53 protein is prolonged by several hours, making it possible to detect protein by immunohistochemical staining using monoclonal antibody.
The p53 mutation or p53 protein overexpression in pancreatic cancer is reported to be 37-76%.9,12,16-26 In our study it was 31.8%, lower than in previous studies. Some studies have reported association between p53 genes and the cell cycle, and have discussed the role of p53 in the carcinogenesis of pancreatic cancer.24 Zhang et al.25 stated that p53 mutation occurs in relatively early stages of carcinogenesis because p53 protein overexpression is observed in intraductal papillary adenocarcinoma. Ruggeri et al.19 reported similar results. However, researchers have proposed various results in the association between p53 mutation and clinicopathologic properties and survival rate. Many researches report that there is no association between p53 mutation and clinicopathologic properties,9,12,18-21,25 while Yokoyama et al.26 reported an association between p53 mutation and clinical stages. In this study, p53 protein overexpression was increased according to the extent of histologic differentiation.
Because some researches report that p53 protein expression is increased according to extent of histologic differentiation and p53 genes control cell cycle, it is probable that p53 mutation is associated with cell differentiation. In effect, further studies will be needed. Although there are many reports showing no relationship between mutation and survival, Yokoyama et al.,26 Nakamori et al.,22 and this study found that subjects with p53 protein overexpression had decreased survival; the varying results are probably due to diverse inclusion criteria and selection bias of the studies. Prospective randomized studies with more subjects are needed to clarify this issue.
Many studies observed pancreatic cancer cases in which p16 and p53 genes showed both variations.27 Naumann et al.28 reported that 77% of patients had variations either in p16 or p53 genes. In this study, although results were not shown, 6 cases (13.6%) had both loss of p16 protein expression and p53 overexpression, and 28 cases (63.6%) had either. However, concurrence of p16 protein loss and p53 overexpression failed to show clinical significance. A genetic model, including several genes associated with carcinogenesis could be applied to pancreatic cancer because many genes besides p16 and p53, such as K-ras and DPC4, have been implicated. The interactions between such genes has been identified to be related to carcinogenesis. In effect, further studies, including investigation of other tumor suppressor genes or oncogenes apart from p16 and p53, will contribute to the treatment of pancreatic cancer.
Figures and Tables
Fig. 1
Results of immunohistochemical staining of p16 and p53. Loss of p16 expression was noted in 20 (45.5%) patients and overexpression of aberrant p53 protein was noted in 14 (31.8%) patients.
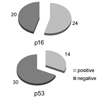
Fig. 2
Positive immunohistochemical staining for p16 protein and aberrant p53 protein. (A) p16 protein expression in pancreatic ductal adenocarcinoma. (B) aberrant p53 protein expression in pancreatic ductal adenocarcinoma (A and B, LSAB ×200).
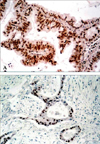
Fig. 3
Disease-free survival of patients with pancreatic ductal adenocarcinoma according to p16 protein expression.

Fig. 4
Disease-free survival of patients with pancreatic ductal adenocarcinoma according to aberrant p53 protein expression.

References
1. Ministry of Health and Welfare, Republic of Korea. Annual Report of the Central Cancer Registry in Korea (1997.1.1~1997.12.31). 1999.
2. Yeo CJ, Cameron JL. Pancreatic cancer. Curr Probl Surg. 1999. 36:59–152.
3. Crist DW, Cameron JL. The current status of the Whipple operation for periampullary carcinoma. Adv Surg. 1992. 25:21–49.
4. Fernandez-del Castillo C, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg. 1995. 130:295–300.
5. Yoon DS, Jeong J, Park YN, Kim KS, Kwon SW, Chi HS, et al. Expression of biliary antigen and its clinical significance in hepatocellular carcinoma. Yonsei Med J. 1999. 40:472–477.
6. Caldas C, Hahn SA, da Costa LT, Redston MS, Schutte M, Seymour AB, et al. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet. 1994. 8:27–32.
7. Redston MS, Caldas C, Seymour AB, Hruban RH, da Costa L, Yeo CJ, et al. p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Cancer Res. 1996. 54:3025–3033.
8. Goggins M, Schutte M, Lu J, Moskaluk CA, Weinstein CL, Petersen GM, et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996. 56:5360–5364.
9. Rozenblum E, Schutte M, Goggins M, Hahn SA, Panzer S, Zahurak M, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997. 57:1731–1734.
10. Huang L, Goodrow TL, Zhang S, Klein-Szanto AJP, Chang H, Ruggeri BA. Deletion and mutation analyses of the p16/MTS1 tumor suppressor gene in human ductal pancreatic cancer reveals a higher frequency of abnormalities in tumor derived cell lines than in primary ductal adenocarcinomas. Cancer Res. 1996. 56:1137–1141.
11. Hu YX, Watanabe H, Ohtsubo K, Yamaguchi Y, Ha A, Okai T, et al. Frequent loss of p16 expression and its correlation with clinicopathological parameters in pancreatic carcinoma. Clin Cancer Res. 1997. 3:1473–1477.
12. Kawesha A, Ghaneh P, Andren-Sandberg A, Ograed D, Skar R, Dawiskiba S, et al. K-ras oncogene subtype mutations are associated with survival but not expression of p53, p16INK4A, p21WAF-1, cyclin D1, erbB-2 and erbB-3 in resected pancreatic ductal adenocarcinoma. Int J Cancer. 2000. 89:469–474.
13. Moskaluk CA, Hruban RH, Kern SE. p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res. 1997. 57:2140–2143.
14. Wilentz RE, Geradts J, Maynard R, Offerhaus GJ, Kang M, Goggins M, et al. Inactivation of the p6 (INK4A) tumor-suppressor gene in pancreatic duct lesions: loss of intranuclear expression. Cancer Res. 1998. 58:4740–4744.
15. Bartsch D, Shevlin DW, Callery MP, Norton JP, Wells SA Jr, Goodfellow PJ. Reduced survival inpatients with ductal pancreatic adenocarcinomas associated with CDKN2 mutation. J Natl Cancer Inst. 1996. 88:680–682.
16. Eskelinen MJ, Haglund UH. Prognosis of human pancreatic adenocarcinoma: review of clinical and histopathological variables and possible uses of new molecular methods. Eur J Surg. 1999. 165:292–306.
17. Sakorafas GH, Tsiotou AG, Tsiotos GG. Molecular biology of pancreatic cancer; oncogenes, tumour suppressor genes, growth factors, and their receptors from a clinical perspective. Cancer Treat Rev. 2000. 26:29–52.
18. Dergham ST, Dugan MC, Kucway R, Du W, Kamarauskiene DS, Vaitkevicius VK, et al. Prevalence and clinical significance of combined K-ras mutation and p53 aberration in pancreatic adenocarcinoma. Int J Pancreat. 1997. 21:127–143.
19. Ruggeri BA, Huang L, Berger D, Chang H, Klein-Szanto AJ, Goodrow T, et al. Molecular pathology of primary and metastatic ductal pancreatic lesions. Cancer. 1997. 79:700–716.
20. Gansauge F, Gansauge S, Schmidt E, Muller J, Beger HG. Prognostic significance of molecular alterations in human pancreatic carcinoma-an immunohistological study. Langenbecks Arch Surg. 1998. 383:152–155.
21. Nio Y, Dong M, Iguchi C, Yamasawa K, Toga T, Itakura M, et al. Expression of Bcl-2 and p53 protein inresectable invasive ductal carcinoma of the pancreas: effects on clinical outcome and efficacy of adjuvant chemotherapy. J Surg Oncol. 2001. 76:188–196.
22. Nakamori S, Yashima K, Murakami Y, Ishikawa O, Ohigashi H, Imaoka S, et al. Association of p53 gene mutations with short survival in pancreatic adenocarcinoma. Jpn J Cancer Res. 1995. 86:174–181.
23. Campani D, Boggi U, Cecchetti D, Esposito I, Ceccarelli F, D'Antonio L, et al. p53 overexpression in lymph node metastases predicts clinical outcome in ductal pancreatic cancer. Pancreas. 1999. 19:26–32.
24. Lynch HT, Brand RE, Lynch JF, Fusaro RM, Kern SE. Hereditary factors in pancreatic cancer. J Hepatobiliary Pancreat Surg. 2002. 9:12–31.
25. Zhang SY, Ruggeri B, Agarwal P, Sorling AF, Obara T, Ura H, et al. Immunohistochemical analysis of p53 expression in human pancreatic carcinomas. Arch Pathol Lab Med. 1994. 118:150–154.
26. Yokoyama M, Yamanaka Y, Friess H, Buchler M, Murray K. P53 expression in human pancreatic cancer correlates with enhanced biological aggressiveness. Anticancer Res. 1994. 14:2477–2483.
27. Pancreas Club Meeting. May 19, 1996, San Francisco, California. Am J Surg. 1997. 173:153–158.
28. Naumann M, Savitskaia N, Eilert C, Schramm A, Kalthoff H, Schmiegel W. Frequent codeletion of p16/MTS1 and p15/MTS2 and genetic alterations in p16/MTS1 in pancreatic tumors. Gastroenterology. 1996. 110:1215–1224.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download