This article has been
cited by other articles in ScienceCentral.
Abstract
Serum rheumatoid factor (RF) is important in the diagnosis and prognosis of rheumatoid arthritis (RA). The purpose of this study is to compare the clinical characteristics and treatment patterns of RA according to the presence of RF in Korean patients. A retrospective analysis was performed on the records of 109 patients who were followed for at least 2 years, among 230 RA patients who visited at the rheumatology clinic in Ajou University Hospital and who fulfilled the 1987 revised American College of Rheumatology criteria for RA. Sixty-four patients were RF positive (58.7%) and 91 patients were female (83.5%). There was no significant difference in demographic characteristics, joint involvements, or percentage of morning stiffness between seropositive and seronegative groups. Anti-nuclear antibody was detected more frequently in the seropositive group (p<0.05). At initial diagnosis, the seropositive group had higher white blood cell and platelet counts than the seronegative group (p<0.01). However, the difference was disappeared at the last follow-up. Inflammatory markers such as ESR and CRP were also higher at diagnosis in the seropositive group (p<0.01). These inflammatory markers were still greater than the seronegative group at the last follow-up (p<0.01). There was no significant difference in the use of disease modifying antirheumatic drug (DMARD) and steroid dosage between groups. However, DMARD combination therapy was more commonly used in the seropositive group (p<0.05), especially triple DMARD combination. These results suggest that disease activity is more severe in the seropositive than the seronegative group, and more aggressive treatments are needed in the seropositive group.
Go to :

Keywords: Rheumatoid arthritis, rheumatoid factor, inflammation, antirheumatic drug, combination therapy
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic inflammatory disease that predominantly manifests as persistent synovial inflammation of peripheral joints. Severity and prognosis of RA are influenced by a variety of demographic factors, such as race, gender, age, profession, and educational level. Clinical factors, such as symptom duration, number of involved joints, rheumatoid nodule, systemic manifestations, and radiologic changes at initial diagnosis are important prognostic factors.
1,
2 Also, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and rheumatoid factor (RF) are useful laboratory findings affecting the prognosis of RA.
2,
3
RF is an antibody against the Fc portion of immunoglobulin G. RF was first described by Waaler and Rose in 1940,
4 and Pike stated in 1949 that RF could be utilized as a diagnostic criteria in RA.
5 RF is observed not only in RA, but also in other rheumatic diseases like Sjogren's syndrome, systemic lupus erythematosus, polymyositis and dermatomyositis, and in inflammatory diseases such as chronic hepatitis. Even in healthy people, RF levels increase with age, and positive reactions can be seen in 5% of young people and up to 25% of the elderly. RF is an important laboratory parameter because RF positive RA patients have more frequent joint deformity and extra-articular manifestation than RF negative patients. Also, the possibility of developing RA is high in healthy people with RF.
6,
7
In this study, we tried to make a retrospective comparison of clinical and laboratory characteristics and treatment patterns according to RF status at diagnosis.
Go to :

MATERIALS AND METHODS
The clinical characteristics of 109 RA patients whose follow-up period was more than two years were studied retrospectively from a total of 230 patients with RA who were cared for at the rheumatology clinic in Ajou University Hospital from June 1995 to March 2002. At the time of diagnosis, the patients satisfied the 1987 revised American College of Rheumatology criteria for classification of RA. Patients diagnosed before 16 years of age were excluded. Patients with arthritis due to other disease, such as gout, ankylosing spondylitis, Reiter's syndrome, psoriasis, inflammatory bowel disease, systemic lupus erythematosus, Behçet's disease, and adult onset Still's disease were also excluded.
Patient records were reviewed and a standard form was used for all relevant clinical information on demographic, clinical, laboratory, and therapeutic characteristics from the time of diagnosis until the end of the study period. All clinical information was entered into a computer database. The patients were divided into seropositive and seronegative groups. A patient was considered seropositive if the IgM RF test result was higher than 40IU/ml and seronegative if IgM RF was less than that.
The following parameters were recorded for each patient at the time of diagnosis: age, gender, duration of symptoms before diagnosis, length of follow-up, family history of RA, morning stiffness, and distribution of involved joints. The following extraarticular manifestations during the disease period were also noted: rheumatoid nodule, vasculitis, serositis, episcleritis, and sicca symptoms. We documented ESR, CRP, hematocrit, white blood cell (WBC) count, and platelet count at the initial diagnosis and at the last follow-up. Also, ANA result was verified. The patients had X-rays of their hands and feet taken at their first visit, and some patients had follow-up X-rays. These were reviewed using the Steinbrocker grading system (grades I-IV) by one radiologist.
With regard to the treatment, we recorded the use of NSAID, the use of each disease modifying anti-rheumatic drug (DMARD), and the incidence of DMARD combination treatment. We used DMARD combination therapy in double and triple DMARD combinations according to the disease severity assessed by the patients symptoms (morning stiffness and visual analogue pain scale), physical findings (tender joint count and swollen joint count), and inflammation markers (ESR and CRP). Finally, steroid usage was measured in two ways; mean steroid dose, which was calculated by dividing the total amount of steroid (mg of prednisone equivalent) used during the study period by the days of disease duration, and maximum steroid dose, which was the highest daily steroid dose used during the study period.
For statistical analysis of clinical and laboratory data derived from the patient groups, SPSS version 10.0 (Statistical Package for the Social Sciences, Chicago, IL) was utilized for descriptive statistical analysis, χ2-test, and independent t-test. Results were specified as means with standard deviation. A p-value of 0.05 or below was regarded as significant.
Go to :

RESULTS
Clinical features
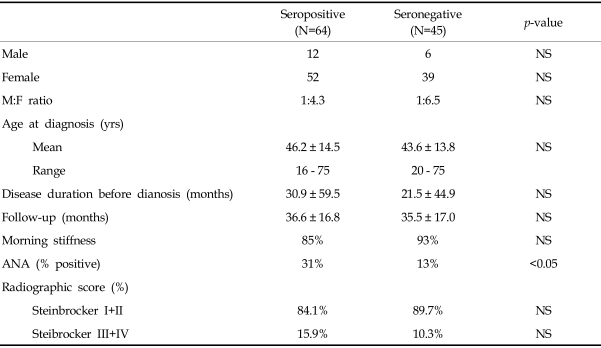
Among the 109 patients, 64 (58.7%) were seropositive and 45 (41.3%) were seronegative (
Table 1). In the seropositive group, the male-female ratio was 1:4.3, with 12 men and 52 women. The male-female ratio was 1:6.5 in the seronegative group, with 6 men and 39 women. The mean age at diagnosis of RA was 46.2 ± 14.5 years (range: 16-75 years) for the seropositive group and 43.6 ± 13.8 years (range: 20-75 years) for the seronegative group (
Fig. 1). Regarding symptom duration before diagnosis, the seropositive group reported 30.9 ± 26.8 months, while the seronegative group reported 21.5 ± 21.1 months. This long symptom duration is probably related to the fact that our clinic is a tertiary referral hospital. Total follow-up period was 36.6 ± 16.8 and 35.5 ± 17 months, respectively. Regarding morning stiffness, there was no statistically significant difference between groups, as it was present in 85% and 93% of patients, respectively. There was no difference in the total number of involved joints and both groups showed nearly identical frequency of joint involvement in the following order; finger joints [metacarpophalangeal (MCP) joints and proximal interphalangeal (PIP) joints], knee joints, elbow joints and shoulder joints, wrist joints, ankle joints, and toe joints (
Fig. 2). Only ankle joints were more frequently involved in the seropositive group (
p<0.05). Fourteen seropositive and 5 seronegative patients had family history of RA. The number of patients with sicca syndrome was 8 of 64 in the seropositive group and 8 of 45 in the seronegative group. Regarding cases of rheumatoid nodule, there were only 3 and 2 respectively from each group, which was statistically insignificant. The incidence of extraarticular manifestation may be lower because of the retrospective nature of this study.
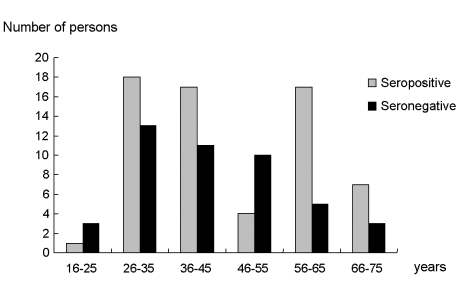 | Fig. 1Age distribution of rheumatoid factor positive and negative rheumatoid arthritis patients. 
|
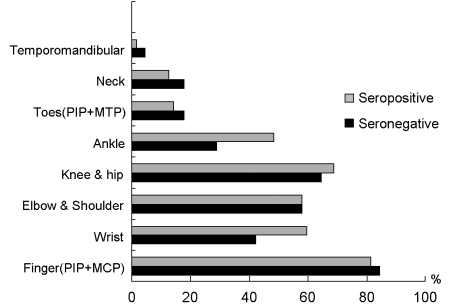 | Fig. 2Involved joints according to rheumatoid factor status. 
|
Table 1
Demographic Characteristics of the Study Subjects

Laboratory features
There was significant difference in ANA positive results according to RF status (
Table 1). Thirty-one percent of the seropositive group were positive for ANA, but only 13% in the RF negative group (
p<0.05). Hematocrit level at initial diagnosis and last follow-up was not different between seropositive and seronegative groups (
Fig. 3). In each group, WBC counts were 8045 ± 2543/µL and 6836 ± 1957/µL respectively (
p<0.01) at diagnosis, and 7154 ± 2397/µL and 6436 ± 1908/µL, respectively (
p=0.09) at the final follow-up. Also, platelet counts showed 305 ± 81.7×10
3/µL and 264.4 ± 83.2×10
3/µL, respectively (
p<0.01) at diagnosis, and 275.9 ± 80.5×10
3/µL and 257.9 ± 72.3×10
3/µL, respectively (
p=0.23) at the final follow-up. At the beginning of RA, seropositive patients showed higher WBC counts and platelet counts as a marker of acute inflammation, but the difference gradually disappeared with treatment. During the entire follow-up period, cumulative inflammation was higher in the seropositive than the seronegative group.
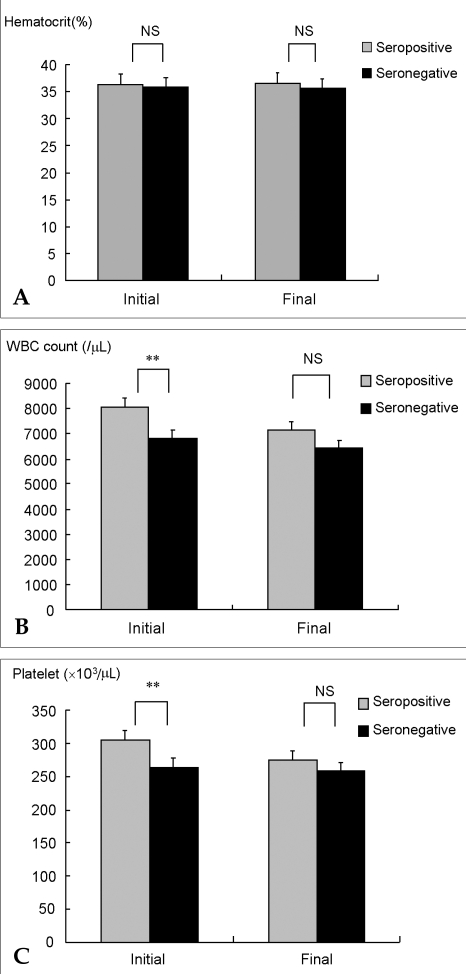 | Fig. 3Hematologic cell counts according to rheumatoid factor status. (A) Hematocrit levels were not different between seropositive and seronegative groups; (B) white blood cell (WBC) counts were higher in the seropositive group at diagnosis, but differences between groups disappeared at the final follow-up; (C) platelet counts mimicked the WBC trend. *p<0.05, **p<0.01, NS: not significant. 
|
The ESR at diagnosis was 49.1 ± 36.8 mm/hr and 26.6 ± 24.3 mm/hr (
p<0.01) in seropositive and seronegative groups, respectively (
Fig. 4). Following treatment, ESR levels gradually decreased to 26.1 ± 20.8 mm/hr and 16 ± 9.4 mm/hr, respectively (
p<0.01) at the final follow-up. The same trend was noted in CRP levels; 2.9 ± 4.1 mg/dL and 0.8 ± 1.2 mg/dL (
p<0.01) was observed at diagnosis, while 0.6 ± 1.1 mg/dL and 0.2 ± 0.4 mg/dL (
p<0.01) was observed at the final follow-up in seropositive and seronegative groups, respectively. These results revealed that the seropositive group had more inflammation than the seronegative at diagnosis and throughout the follow-up period. The treatment of RA could decrease but not eliminate the inflammation, as the seropositive group had more inflammation than the seronegative at the last follow-up.
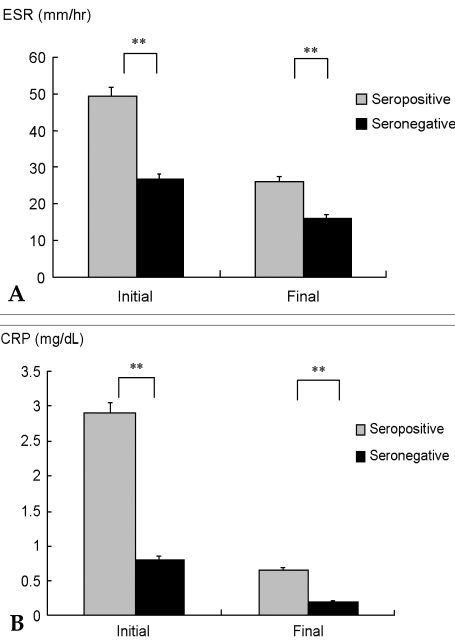 | Fig. 4Inflammatory markers according to rheumatoid factor status. (A) erythrocyte sedimentation rate (ESR) was higher in the seropositive group at diagnosis and throughout the follow-up; (B) C-reactive protein (CRP) followed the ESR trend. **p<0.01. 
|
Radiologically, there were no statistically significant differences between groups (
Table 1). However, in the seropositive group, seven patients showed progression on their follow-up X-rays. In the seronegative group, two patients showed progression and one patient showed improvement.
Treatment according to RF status
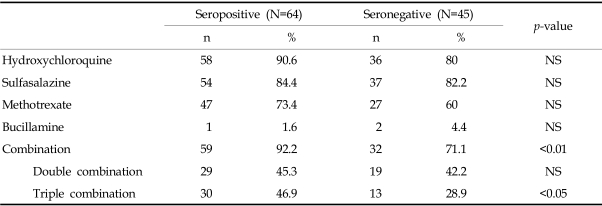
As DMARD, hydroxychloroquine, sulfasalazine, methotrexate, and bucillamine were used during the study period. Hydroxychloroquine was most commonly prescribed in both groups; 58 patients (90.6%) of the seropositive and 36 patients (80%) of the seronegative group (
Table 2). Sulfasalazine and methotrexate were also frequently used; in the seropositive group, 54 patients (84.4%) and 47 patients (73.4%), respectively, and in the seronegative group, 37 patients (82.2%) and 27 patients (60%), respectively. There was no difference in the use of DMARD between groups. However, more patients were prescribed a combination of DMARD in the seropositive than the seronegative group; 59 patients (92.2%) vs. 32 patients (71.1%), respectively (
p<0.003). Triple DMARD combination therapy was also more commonly used in the seropositive group (30 patients, 46.9%) vs. the seronegative group (13 patients, 28.9%) (
p<0.05). There was no difference in the mean steroid dose between the seropositive and seronegative groups (
Fig. 5): 4.07 ± 2.98 mg/day and 3.27 ± 3.01 mg/day, respectively (
p=0.16). Also, the maximum steroid dose of each group was not different: 8.49 ± 4.12 mg/day and 7.5 ± 4.46 mg/day, respectively (
p=0.26). These results showed that the seropositive group was treated with more aggressive treatment such as DMARD combinations, probably due to presenting with more severe clinical disease activity.
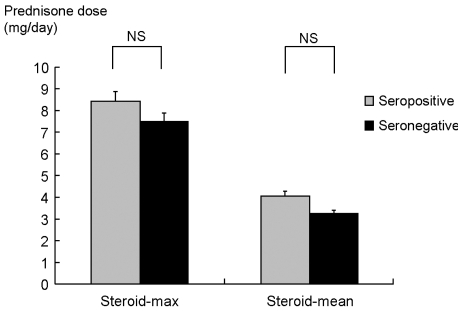 | Fig. 5Comparison of corticosteroid dose according to rheumatoid factor status. Steroid-max: the highest daily steroid dose (mg of prednisone equivalent) used during study period; Steroid-mean: total amount of steroid used during study period divided by the days of disease duration; NS, not significant. 
|
Table 2
DMARD Usage According to Rheumatoid Factor Result

Go to :

DISCUSSION
RA is a chronic inflammatory disease that has various clinical courses. Prognosis is influenced by demographic factors, clinical factors, and radiologic changes at initial diagnosis. Also, ESR, CRP, and RF at diagnosis are laboratory factors that affect the prognosis of RA.
1-
3 We planned to evaluate the clinical characteristics and treatment strategy according to RF status in Korean RA patients, because the clinical manifestations and prognosis of RA could be different upon race.
8 Moreover, there had been no such study in Korea to date. In this study, there was no difference in clinical characteristics such as gender, age at diagnosis, symptom duration before diagnosis, follow-up period, and presence of morning stiffness according to RF status. Joint involvement was not different, except that the ankle joint was more frequently affected in the seropositive group. From a study by Papadopoulos et al. in Greece, male-female ratio, age at diagnosis, disease duration, and morning stiffness were not significantly different between seropositive and seronegative groups. However, seropositive patients had a longer follow-up period, more frequent involvement of hand joint, and higher involved joint counts than the seronegative group.
9 Furthermore, a report indicated that rheumatoid nodule was highly reported in seropositive patients.
10 When Van de Heijde et al. checked the factors related to poor prognosis in RA, they found that woman, RF positive status, high ESR and CRP, anemia, and rheumatoid nodule were associated with poor outcomes.
3
We noticed more severe inflammation in the seropositive group as well as increased ESR and CRP levels at diagnosis and continuously during follow-up. Also, WBC and platelet counts were higher in seropositive patients at diagnosis, however they were not at last follow-up. It could be because the inflammation decreased due to DMARD treatment and cell counts were less sensitive to inflammatory markers such as ESR and CRP. Amos et al. reported that RA patients with high ESR and CRP have serious radiological changes including bony erosion, regardless of RF status.
11 It was revealed in a further study that the seropositive group shows a higher inflammation degree, lower hemoglobin level, and increased WBC and platelet levels than the seronegative group.
12,
13 Regarding limitations of this retrospective study, we couldn't measure the radiologic changes of all study patients. However, there were no statistically significant differences between groups except that all RF positive patients showed progression on follow-up X-rays.
In our Korean RA patients, 31% of seropositive patients showed positive ANA results, while only 13% of the seronegative group did. Though only a few studies regarding the appearance of ANA in RA have been made so far, a Japanese report showed that 35% of RA patients were ANA positive in a speckled or homogeneous pattern.
14 Paulus et al. reported that 41% of American early seropositive RA patients had positive ANA results and higher disease activity.
15 Also, Masi et al. stated that an ANA positive group demonstrated a higher degree of bony erosion, which resulted in poor prognosis of RA.
10 In another report, RF as well as ANA occurred more frequently in nodular RA, with serious progress of extraarticular symptoms and radiologic changes.
16 There was also a report indicating that the level of immunoglobulin was elevated in serum and synovial fluid to a greater degree in seropositive patients.
17 These results and ours suggest that more autoimmune and inflammatory responses are present in seropositive RA patients.
Regarding RA treatment according to RF presence, Papadopoulos et al. reported a high frequency of hydroxychloroquine, D-penicillamine, and methotrexate in seropositive patients.
9 In a prospective study for an average of 6.2 years, Mottonen et al. reported that RA patients used 3.3 DMARDs on average.
18 However, as far as we know, there has been no study made on the frequency of DMARD combination therapy according to RF status. Though each DMARD did not show any difference in usage frequency according to RF status, the frequency of DMARD combination therapy, especially triple combination therapy, was significantly higher in the seropositive group. From this fact, we infer that the seropositive group might need more DMARDs to control the more severe joint inflammation.
By clinical characteristics and laboratory findings, we verified that more autoimmune and inflammatory responses were present in the seropositive group. The high frequency of DMARD combination therapy in treatment, and still higher inflammatory markers at the last follow-up in the seropositive group, revealed that seropositive patients need more DMARD than seronegative patients, but not enough to control joint inflammation. More aggressive treatment is needed to control disease activity in seropositive RA patients.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download