Abstract
Chylopericardium is a rare clinical entity in which chylous fluid accumulates in the pericardial cavity. We report a case of primary idiopathic chylopericardium associated with multiple, small cervicomediastinal cystic hygromas occurring in an asymptomatic 43-year-old woman with no history of trauma, thoracic surgery, malignancy, infection or tuberculosis. Echocardiography showed a large amount of pericardial effusions and pericardial fluid analysis revealed inappropriately elevated triglyceride. We did not demonstrate communication between the thoracic duct and the pericardial sac by lymphangiography and chest computed tomography. She successfully responded to 30 days of continuous pericardial drainage and 15 days of a medium-chain triglyceride diet after 30 days of total parenteral nutrition. Follow-up echocardiography 6 months after treatment commencement showed a minimal reaccumulation of pericardial fluid without symptom. We conclude that if a patient is asymptomatic and can well tolerate daily life, surgery including pericardiectomy or ligation of the thoracic duct is not necessarily required.
The accumulation of chyle in the pericardial cavity, therefore called chylopericardium, is an unusual clinical entity.1 It usually arises from mediastinal neoplasma,2 thrombosis of the subclavian vein,3 tuberculosis,4 nonsurgical trauma5 or thoracic or cardiac surgery.6,7 Primary idiopathic chylopericardium, which literally means it has no known etiology, is even rarer.8-16 It was first described as clinical entity by Groves and Effler in 1954.8 Although several reviews on the subject have been published, its etiology remains unclarified. However, many previously reported cases of primary idiopathic chylopericardium were treated by ligating and resecting the thoracic duct, which suggests that leaks from the thoracic lymphatics resulted in the chylous effusion.8,10,11,13-16 We report a case of primary idiopathic chylopericardium associated with multiple, small cystic hygromas in a 43-year-old woman, which was successfully treated using pericardiostomy, concomitantly with medium chain triglyceride and low fat meal dietary support after total parenteral nutrition.
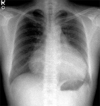
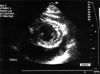
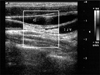
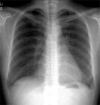
A 43-year-old woman was referred to our hospital for cardiomegaly on chest X-ray (Fig. 1). Physical examinations revealed a healthy appearing woman except for distant heart sounds. All laboratory findings were within the normal range. The patient had no history of trauma, congenital heart disease, thoracic surgery or tuberculosis. Transthoracic echocardiography revealed a large amount of pericardial effusion without hemodynamic compromise. Diagnostic pericardiocentesis was performed with 500 mL of a milky-colored pericardial fluid aspirated. Routine examination of the pericardial fluid revealed pH 6.8, protein 5.0 g/dL, and LDH 234 IU/L. WBCs and RBCs were uncountable due to cell clumping. Routine bacterial and tuberculous cultures were subsequently reported as negative, as was the cytological examination, and the adenosine deaminase level was 15.5 U/L. Considering these clinical and laboratory findings, the initial clinical impression was of nonspecific chronic pericarditis with effusion. We did not diagnose chylopericardium because we overlooked the clue offered by the color of the pericardial fluid. After pericardiocentesis, follow-up echocardiography revealed that minimal pericardial effusion remained. She was regularly followed up at our outpatient clinic after discharge. However, echocardiography one month later revealed a large amount of pericardial effusion without evidence of hemodynamic compromise (Fig. 2), and she was readmitted to our hospital for further evaluation. The patient, who was afebrile on admission, complained only of mild substernal discomfort. Her blood pressure was 90/60 mmHg and pulse rate 72 beats per minute. On physical examination neck veins were distended and peripheral pulses were normal. Her breathing sound was clear without rale, though heart sounds were slightly decreased and pericardial friction rub was inaudible. The chest X-ray showed marked enlargement of the cardiac shadow with a globar shape, and an electrocardiogram demonstrated a sinus rhythm, with low voltage QRS complexes in the precordial leads. The laboratory examination showed; white cell count 7,740/mm3, hemoglobin 13.0 mg/dL, hematocrit 39%, and platelets 238,000/mm3. The ESR was 17 mm/hr and CRP 6.7 mg/dL. Blood chemistry revealed; BUN 12.9 mg/dL, creatinine 0.6 mg/dL, total protein 7.0 g/dL, albumin 4.7 g/dL, AST/ALT 21/17 IU/L, total cholesterol 196 mg/dL, triglyceride 111 mg/dL, HDL-cholesterol 46 mg/dL, and LDL cholesterol 138 mg/dL. Urinalysis was normal. Antinuclear antibody and anti-viral antibodies including HIV were all negative. β2-microglobulin and CEA were within the normal range. Repeated pericardiocentesis was performed, and an immediate gushing out of 700 mL of a milky-yellowish fluid ensued (Fig. 3). An examination of the pericardial fluid showed pH 6.8, protein 7.0 g/dL, glucose 98 mg/dL, LDH 560 IU/L, cholesterol 97 mg/dL, and triglyceride 1078 mg/dL. RBCs and WBCs were uncountable due to cell clumping. The adenosine deaminase level was 17.0 U/L and the CEA level 1.15 ng/mL. Routine cultures for bacteria and tuberculosis were subsequently reported as negative and a cytologic examination showed no tumor cells. These findings with inappropriately elevated triglyceride in the pericardial fluid sufficed to diagnose chylopericardium. The patient underwent extensive investigations to determine the secondary causes. Chest computed tomography revealed a suspicious small cystic lesion on the left neck and mediastinal haziness. The possibility of cystic hygroma with mediastinal extension was highly suggested by radiologists. Neck sonography also showed a 1.9 cm sized multilocular, compressible cystic lesion between the sternocleidomastoid muscle and the internal jugular vein of the left neck. These findings were compatible with cystic hygroma (Fig. 4). The cystic lesion could not be aspirated due to patient's refusal. To demonstrate the presence of any anomalous communication between the lymphatic channels and the pericardial sac, we performed a direct lymphangiography. However, no direct communication between the pericardial sac and the thoracic duct could be definitely established. Follow-up echocardiography was performed 10 days after the pericardiocentesis and showed a massive reaccumulation of pericardial effusion. 11 days after pericardiocentesis, the patient underwent pericardiostomy. Using the subxyphoid approach, a pericardial window was made with 32 Fr draining tube insertion in an attempt to prevent pericardial fluid reaccumulation and cardiac tamponade. Incision of the pericardium resulted in the extrusion of 600 mL or more of milky fluid. The pericardial fluid analysis showed that it had the same nature as the previous sample. When the patient was placed on total parenteral nutrition for 30 days and a subsequent low fat diet enriched with medium-chain triglycerides for 15 days, the drainage reduced to 30-40 mL per day. On the 45th day after pericardiostomy, drainage tube was removed and she was discharged from the hospital on a low fat diet. She remained totally asymptomatic without interval changes of heart size on chest X-ray (Fig. 5) or clinical signs of cardiac tamponade at the 6 month follow-up.
Groves and Effler,8 in 1954, reported a case of chylopericardium in a 31-year-old woman who was found to have an isolated recurrent accumulation of chyle in the pericardium associated with mediastinal cystic hygroma. They used the term "primary idiopathic chylopericardium" in this first case report because no apparent etiologic mechanism was evident. This is such a rare condition that a computerized Medline search from 1960 to 2003 identified fewer than 35 cases of primary idiopathic chylopericardium, including our case. Its etiology is obscure, though in recent years, a few reports have described a lymphatic leak and fistula by combined lymphangiography and computed tomography or intraoperative thoracic ductogram.9,14 These reports clearly demonstrate a leaking flow of contrast media from the thoracic duct to the pericardial sac. This disorder has two possible explanations: (1) the presence of damaged lymphatic valves and the communication of thoracic duct to the pericardial lymphatics resulting in chylous reflux and (2) abnormally elevated pressure in the thoracic duct observed as lymphangiectasia.14,15
Diagnostic modalities essential for evaluating suspected chylopericardium include the following: A chest X-ray demonstrating cardiomegaly will lead to further investigations and eventually diagnosis. Pericardial effusion is usually diagnosed by echocardiography or computed tomography. The nature of the effusion is usually revealed by pericardiocentesis.9 Computed tomography and lymphangiography may be useful in the diagnosis of mediastinal lymphangiomatosis, which is the most common cause of primary chylopericardium.17 Investigation specifically targeting malignant disease, lymphoma and tuberculosis should also be undertaken.2,4 A history of trauma caused by thoracic surgery,6,7 the introduction of a subclavian venous catheter,3 or blunt trauma5 should be determined. Lymphangiography may in certain instances establish fistulous connections and is also useful for delineating the anatomy of the thoracic duct.9,14,21 Primary chylopericardium should be considered in the differential diagnosis of chronic pericardial effusion. As is pointed out in a review by Akamatsu,14 about a half of patients are asymptomatic or were noted to have only cardiomegaly by chest X-ray. Cystic hygromas (lymphangiomas) may arise from lymphatic tissue normally present in the area or may grow directly from mesodermal rests that produce imperfect lymphoid channels.18 These lesions are usually found in the neck or axilla. It is apparent that lymphangioma may be multifocal, involving mediastinum, retroperitoneum, and even the skeletal system, and that they produce a number of apparently separate syndromes depending on the anatomic location of the lesion.18-20 Our patient had cystic lesion in the neck, but she refused biopsy for a definitive diagnosis. However, according to the smoothly marginated cystic mass on CT scan and the compressibility of the lesion by sonography, we concluded that this cystic lesion was a cystic hygroma. In our case, we failed to visualize any communication between the thoracic duct and the pericardial sac by lymphangiography and computed tomography of the chest. Tucker et al.20 in 1967 reported idiopathic chylothorax in association with a cystic lesion of the bone, and Goldstein et al.18 in 1969 reported an association of a chylopericardium with multiple lymphangioma of the bone. Moreover, although they failed to demonstrate a clear communication between the pericardial space and the thoracic duct, they pointed out a generalized abnormality of the lymphatic system in patients with lymphangiomas. We strongly believe that a cystic hygroma of the neck and chylopericardium reflect a generalized disorder of the lymphatic system in our patient. An abnormal lymphatic system may cause the lymphatic channel pressure elevation and increase the permeability of lymphatic vessels, resulting in the reflux of chyle through certain communications between the lymphatic system and the pericardial sac.18-20
The management of chylopericardium is guided by the prevention of cardiac tamponade, and the avoidance of the metabolic, nutritional, and immunologic consequences that occur given a significant chyle leak, and to reduce the likelihood of recurrence.8-16 Several different approaches make be adopted for the treatment of chylopericardium: (1) pericardiocentesis, (2) thoracostomy drainage, (3) dietary support with medium or short chain triglyceride and low fat meals, (4) pericardiectomy, (5) pericardial window formation, (6) ligation of the thoracic duct above the level of the diaphragm, and (7) a pericardial-peritoneal (Denver) shunt. In contrast to post-traumatic chylopericardium, the conservative treatment of idiopathic chylopericardium is rarely successful. Surgical treatment is usually required, however, it should be noted that only one case treated conservatively has been reported.12 Nevertheless, initially, a patient should be treated conservatively by pericardial drain placement and the institution of a medium chain triglyceride diet or total parenteral nutrition.10 If this approach fails, then surgical ligation of the thoracic duct just above the diaphragm combined with window formation or pericardiectomy to ensure adequate drainage has been reported to be definitive treatment.8-11,13-15 Pericardial drainage with pericardiocentesis or tube thoracostomy with or without dietary management was also found effective, but in many cases reaccumulation occurred.9 Pericardio-peritoneal shunt placement has been advocated by Chan et al.22 as a simple and effective alternative to prolonged pericardial drainage or thoracotomy. Although most authors reported surgery as the definitive method of treatment, Lopez-Castilla et al.12 reported the successful treatment of a 2-month-old boy by low fat total parenteral nutrition. In our patient, external drainage of the pericardial sac via the pericardiostomy, concomitant with dietary support with medium chain triglyceride and low fat meals, resulted in the complete resolution of pericardial effusion. Six months later, the patient was asymptomatic and the chest X-ray showed a normal cardiac size. This success in our case reasserts the importance of conservative treatment for the management of primary idiopathic chylopericardium. If symptoms develop or massive pericardial effusion recurs, thoracic duct ligation will be considered in this patient.
In summary, our case supports that pericardiostomy combined with total parenteral nutrition and a low fat dietary support is a treatment modality for primary idiopathic chylopericardium
Figures and Tables
References
1. Crosby IK, Crouch J, Reed WA. Chylopericardium and chylothorax. J Thorac Cardiovasc Surg. 1973. 65:935–939.
2. Barton JC, Durant JR. Isolated chylopericardium associated with lymphoma. South Med J. 1980. 73:1551.
3. Smedts F, Kubat K, Chande H. Chylopericardium and chylothorax, resulting from a catheter to the left subclavian vein: an autopsy report. Klin Wochenschr. 1989. 67:1214–1217.
4. Lorell BH, Braunwald E. Braunwald E, editor. Pericardial disease. Heart disease. A textbook of cardiovascular medicine. 1992. Philadelphia: W.B. Saunders;1505.
5. DeMeester TR, Lafontaine E. Sabiston DCJ, Spencer FC, editors. The pleura. Gibbon's Surgery of the chest. 1983. Philadelphia: W.B. Saunders;369–372.
6. Delaney A, Daicoff GR, Hess PJ, Victorica B. Chylopericardium with cardiac tamponade after cardiovascular surgery in two patients. Chest. 1976. 69:381–383.
7. Bar-el Y, Smolinsky A, Yellin A. Chylopericardium as a complication of mitral valve replacement. Thorax. 1989. 44:74–75.
8. Groves LK, Effler DB. Primary chylopericardium. N Engl J Med. 1954. 250:520–523.
9. Yoon YS, Shim WH, Chung TS, Lee YS. Primary idiopathic chylopericardium: Report of a case and review of the literature. Yonsei Med J. 1993. 34:98–108.
10. Ossiani MH, McCauley RGK, Patel HT. Primary idiopathic chylopericardium. Pediatr Radiol. 2003. 33:357–359.
11. Svedjeholm R, Jansson K, Olin C. Primary idiopathic chylopericardium- a case report and review of the literature. Eur J Cardiothorac Surg. 1997. 11:387–390.
12. Lopez-Castilla JD, Soult JA, Falcon JM, Munoz M, Santos J, Gavilan JL, et al. Primary idiopathic chylopericardium in a 2 month old successfully treated without surgery. J Pediatr Surg. 2000. 35:646–648.
13. Akashi H, Tayama K, Ishihara K, Tanaka A, Fujino T, Okazaki T, et al. Isolated primary chylopericardium. Jpn Circ J. 1999. 63:59–60.
14. Akamatsu H, Amano J, Sakamoto T, Suzuki A. Primary chylopericardium. Ann Thorac Surg. 1994. 58:262–266.
15. Yuksel M, Yildizeli B, Zonuzi F, Batirel HF. Isolated primary chylopericardium. Eur J Cardiothorac Surg. 1997. 12:319–321.
16. Hudspeth AS, Miller HS. Isolated (primary) chylopericardium. J Thorac Cardiovasc Surg. 1966. 51:528–531.
17. de Menezes IC, Araujo eSG, Damiao A, Telo M, Martins FM, Macedo MM. Lymphangioma of the mediastinum as a cause of chylopericardium. Acta Med Port. 1990. 3:119–121.
18. Goldstein MR, Benchimol A, Cornell W, Long DR. Chylopericardium with multiple lymphangioma of bone. N Engl J Med. 1969. 280:1034–1037.
19. Bhatti MAK, Ferrante JW, Gielchinsky I, Norman JC. Pleuropulmonary and skeletal lymphangiomatosis with chylothorax and chylopericardium. Ann Thorac Surg. 1985. 40:398–401.
20. Tucker SM. Bilateral chylothorax with multiple osteolytic lesion?: generalized abnormality of lymphatic system. Proc Roy Soc Med. 1967. 60:17–19.
21. Rankin RN, Raval B, Finley R. Primary chylopericardium: combined lymphangiographic and CT diagnosis. J Comput Assist Tomogr. 1980. 4:869–870.
22. Chan BBK, Murphy MC, Rodgers BM. Management of chylopericardium. J Pediatr Surg. 1990. 25:1185–1189.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download