Abstract
Rifaximin has been reported to be effective for the treatment of hepatic encephalopathy (HE) in Europe. However, it is unknown whether Rifaximin is effective for the treatment of HE in Koreans, therefore we conducted a open-label prospective randomized study to evaluate the efficacy of rifaximin versus lactulose in Korean patients. Fifty-four patients with liver cirrhosis and hepatic encephalopathy were enrolled. Thirty-two patients were randomized to receive rifaximin and 22 to receive lactulose both over a 7-day periods. Before and at the end of treatment, gradation of blood ammonia, flapping tremor, mental status, number connection test (NCT) were performed and estimation of HE indexes determined. Both rifaximin and lactulose were effective in the majority of patients (84.4% and 95.4%, respectively, p=0.315). Blood NH3, flapping tremor, mental status, and NCT was significantly improved by rifaximin and lactulose, and the posttreatment levels of these measures were similar for the rifaximin and lactulose-treated groups, as was the HE index (rifaximin group (10.0→4.2, p=0.000); lactulose group (11.3→5.0, p=0.000)). One patient treated with rifaximin complained of abdominal pain, which was easily controlled. There was no episode of renal function impairment in either treatment group. Rifaximin proved to be as safe and as effective as lactulose for the treatment of Korean patients with hepatic encephalopathy.
Hepatic encephalopathy (HE) is presented clinically as a combination of neuropsychiatric abnormalities characterized by alterations in mental status, personality, intellectual function, and changes in neuromuscular activity. It is frequently observed in those with both acute liver failure and liver cirrhosis.1 The pathogenesis of the syndrome is complex, but ammonia produced by intestinal bacteria is known to play an important role in its pathogenesis. The treatment of HE has focused on reducing both the production and absorption of gut-derived ammonia. Presently, non-absorbable disaccharides and antibiotics are the mainstay of therapy.2,3 However, currently used drugs have several limitations. For example, neomycin, a poorly-absorbed aminoglycoside may cause nephrotoxicity and ototoxicity,3 and lactulose treatment may be complicated by excessive diarrhea and abdominal pain.4,5
Rifaximin (4-deoxy-4'-methylpyrido-(1',2'-1,2)-imidazo-(5,4C)-rifamycin SV) is a derivative of rifamycin, which acts by inhibiting bacterial ribonucleic acid (RNA) synthesis. Rifaximin is virtually unabsorbed after oral administration and exhibits broad spectrum antimicrobial activity against both aerobic and anaerobic gram-positive and gram-negative microorganisms within the gastrointestinal tract.6 During the past decade, several European studies have proved the efficacy of rifaximin for the treatment of HE in Caucasian patients.7-14 However, no similar investigation has been performed in Asian patients.
It has been reported that ethnicity can affect therapeutic response to anti-viral therapies and the long-term prognosis of patients with advanced liver diseases.15-17 In particular, it is not known whether ethnic background affects the effectiveness of rifaximin in the treatment of HE. In Korea, hepatitis B is the major cause of decompensated liver cirrhosis presenting hepatic encephalopathy, in contrast, in Western countries, liver cirrhosis secondary to alcohol abuse predominates.18,19 Alcohol overdose often leads to intestinal bacterial overgrowth which is one of the important precipitating factors of HE.20 Thus etiologic differences may affect the therapeutic efficacy of rifaximin in Korean patients with HE. Therefore, we conducted this prospective, randomized study to determine the efficacy and tolerability of rifaximin versus lactulose for the treatment of HE in Korean patients.
Sixty-four in-patients with episodic HE who were admitted at Yonsei University Medical Center (Seoul, Korea) were firstly enrolled in the study. All patients were affected by decompensated liver cirrhosis and HE, which were diagnosed based on clinical and laboratory findings. Patients showed signs of the first to third degree HE, according to Conn's modification of Parsons-Smith classification,21 and had serum ammonia levels > 75 µmol/L.
The criteria for exclusion from the study were: an age <18 years, the presence of a major neuropsychiatric illness, presence of intestinal obstruction or inflammatory bowel disease, hypersensitivity to rifamycin or disaccharides, a serum creatinine level>twice normal, those that had received loop diuretics, antacids or cathartics within the 12 hour period preceding study commencement, patients that were on antibiotics during the preceding 7 days, and those that had been treated with encephalopathy-causing agents. Women of child-bearing age were excluded if they were not using a method of contraception.
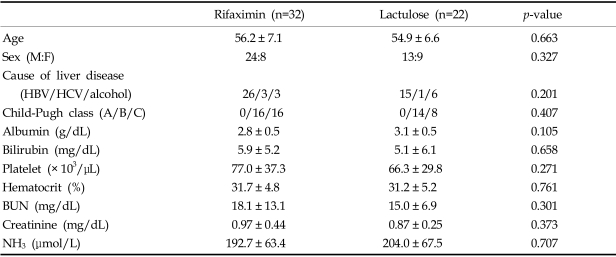
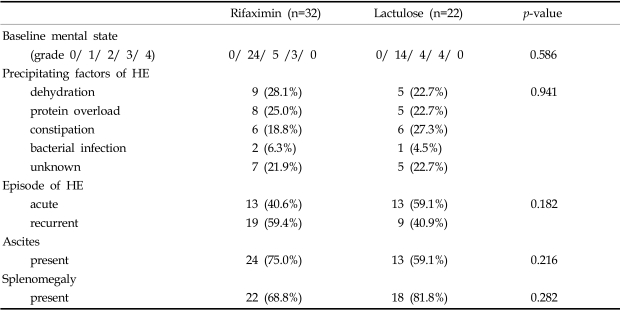
Ten of the 64 initially enrolled patients were excluded, and 54 patients finally entered the trial. The enrolled patients were 37 males and 17 females, aged between 40 and 71 (mean age 55.7 years). At the start of the study, the demographic and clinical parameters of the patients in the two groups showed no significant differences (Table 1). Identified precipitating factors included dehydration (n=14), protein overload (n=13), constipation (n=12), bacterial infection (n=3), and unknown (n=12). There was no significant difference between the two groups in terms of factors precipitating HE (Table 2).
The research was performed in accordance with the revised Helsinki Declaration, and the study protocol adopted was approved by the Yonsei University Medical Center Institutional Ethics Committee. The protocol was explained to at least one relative of each patient selected for the study, and written informed consent was obtained from all patients who participated or from their relatives.
Using a computer-generated sequential 3:2 block randomization list, patients were assigned to receive rifaximin (Normix®, Alfa Farmaceutici SpA, Bologna, Italy) at 1200 mg per day in three divided doses (n=32) or lactulose (Duphalac syrup®, Choongwae Pharmaceutical, Seoul, Korea) at 90 mL a day (n=22). The treatment duration was 7 days unless symptoms worsened or serious side effects occurred. All patients were given a nutritious diet containing a maximum of 40g of protein per day. At the beginning of the trial, patients underwent a full assessment, including a detailed history taking, and physical and neurological examinations. The following parameters were evaluated before and at the end of the treatment period: complete blood count, blood chemistry, serum electrolytes, renal function, blood sugar, and urinalysis. Adverse events were assessed by the investigators. Any abnormal clinical or laboratory findings observed during the trial were monitored and documented. Drug administration was discontinued in the event of 1) severe side effect, 2) taking any medication that could potentially interfere with the course of HE, 3) a serious cirrhotic complication such as acute variceal bleeding, or spontaneous bacterial peritonitis, and 4) a patient's refusal to participate in the trial.
This was examined semi-quantitatively using Conn's modification of the Parsons-Smith classification.21 Grade 0: no abnormality; Grade 1: trivial loss of awareness, euphoria or anxiety, shortened attention span, impairment of addition or subtraction performance; Grade 2: lethargy, disorientation with respect to time, obvious personality change, inappropriate behavior; Grade 3: somnolence to semi-stupor, responsive to stimuli, confusion, gross disorientation, bizarre behavior; and Grade 4: coma, unable to test mental function.
Severity was determined by extending the patients' arms and forearms with the wrists dorsiflexed for at least 30 seconds. We adopted a simplified grading system to minimize inter-observer variance. Grade 0: no flapping motion; Grade 1: infrequent flapping motion; Grade 2: continual flapping motion; and Grade 3: unable to test.
Blood ammonia was measured before and after the treatment using Cobas Integra 800 (Roche, Basel, Switzerland). Grade 0: < 75 µM/L; Grade 1: 76-150 µM/L; Grade 2: 151-200 µM/L; Grade 3: 201-250 µM/L; and Grade 4: > 251 µM/L.
The above items were determined before treatment and on days 3, 5, 7 of the trial period. The grades for the above four components were weighted in proportion to their importance. Thus, HE grade awarded a weighting factor of three, while the other variables were each assigned a factor of one. The HE index was defined as the total of the weighted grades, and had a possible range of 0 to 23 points, i.e., HE index=(grade of mental state) × 3+(grade of number connection test)+(grade of flapping tremor)+(grade of blood ammonia).21
Efficacy was graded as 'improved', 'unchanged', or 'worsened'. A decrease of HE index by at least one point was defined as 'improved', and increment of the HE index by one point or more was defined as 'worsened'.
All data are expressed as means±SD. Statistical evaluations were performed using SAS version 6.12 for Windows (SAS Institute Inc., Cary, NC). Statistical analyses were made using the Student's T-test, the paired T-test, Fisher's exact test, and the Chi-square test. This study satisfied a statistical power of 0.8. A probability level of p < 0.05 was considered to be significant throughout.
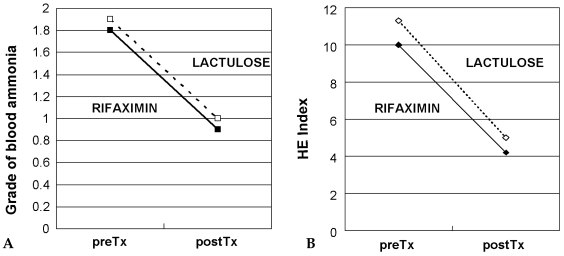
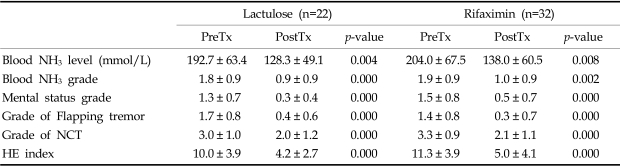
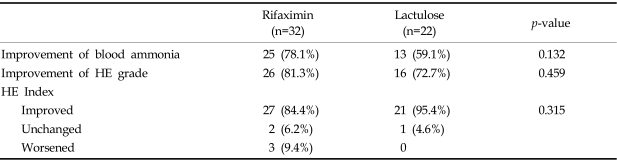
Table 3 compares the therapeutic effects of rifaximin and lactulose. Mean blood levels and grades of blood NH3 significantly decreased with both rifaximin (p < 0.01) and lactulose treatment (p × 0.01). Mean blood NH3 concentrations were similar after both forms of therapy (Fig. 1A). Mental state was significantly improved by rifaximin (1.3→0.3) and by lactulose (1.5→0.5) (p < 0.01 and p < 0.01, respectively). Grades of flapping tremor and NCT were improved to nearly equal degrees by rifaximin and lactulose treatment (Table 3). Mean HE indexes improved both in the rifaximin group (10.0→4.2, p=0.000) and in the lactulose group (11.3→5.0, p=0.000) (Fig. 1B). No significant difference was found between the two groups in terms of the grades of HE components at any given time.
At the completion of treatment, blood NH3 and HE grades improved in 25 (78.1%) and 26 (81.3%), respectively, of the 32 patients in the rifaximin group. In the lactulose group, 13 (59.1%) of the 22 patients showed reduced blood ammonia grades and 16 cases (72.7%) improved HE grades. These group differences were not statistically significant (Table 4). Clinical efficacy was determined using HE index improvement. Rifaximin was considered effective in 27 of 32 patients (84.4%) and lactulose in 21 of 22 patients (95.4%), which was not significantly different (p=0.315) (Table 4).
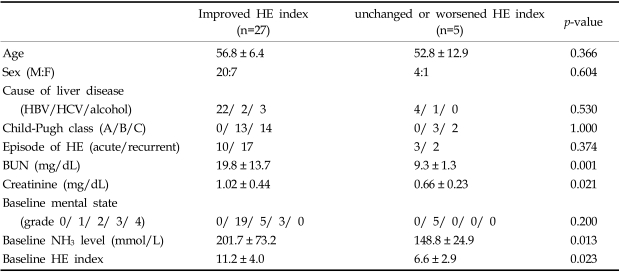
We then compared the clinical characteristics of patients who showed a HE index improvement (n=27) with those who did not (n=5) after rifaximin treatment (Table 5). No significant differences in terms of age, sex, cause of liver disease, Child-Pugh class, episode of HE, or baseline mental state was observed between the two groups. Baseline blood urea nitrogen and creatinine levels were higher in patients who showed HE improvement after rifaximin treatment. Interestingly, the baseline ammonia level and the HE index were higher in the improvement group than in the no-improvement group after rifaximin treatment (p < 0.05) (Table 5).
Overall patient compliance was excellent. One patient treated with rifaximin complained of abdominal pain, and one patient treated with lactulose experienced severe diarrhea, but no patient was withdrawn from the trial due to an undue adverse effect. Renal function impairment did not occur in any patient.
Although a number of other possible factors have been proposed to play a role in the pathogenesis of HE, such as, the production of central benzodiazepine agonists, endogenous opioids and false neurotransmitters, ammonia is still viewed as the key contributor.2 Thus the mainstay treatment for HE revolves about reducing the production and absorption of ammonia in the gut, and to improve its excretion by drug therapy or diet modification. Currently, lactulose and nonabsorbable antibiotics are most commonly used therapeutics to treat HE.1-3 Several placebo-controlled trials of lactulose have reported no proof of superiority versus a placebo. However, these negative results are believed to be due to the designs of trials, variables of efficacy, and to low numbers of enrolled patients.24-27 Lactulose is currently recommended as the first-line pharmacological treatment for HE by the practice guidelines proposed by the American College of Gastroenterology.28 However, the use of lactulose may be associated with nausea, flatulence, abdominal cramps, severe diarrhea, and dehydration.4,5,29 Protracted diarrhea may result in hypertonic dehydration with hypernatremia, which may aggravate the patient's mental state.30
Antibiotics are regarded as a therapeutic alternative to nonabsorbable disaccharides for HE treatment.28 Neomycin is a non-absorbable aminoglycoside that has also been prescribed for HE, but its ototoxicity and nephrotoxicity limit its use in HE.3 Metronidazole, which differs from neomycin in terms of its bacterial spectrum, also improves HE, however its potentially severe neurotoxicity in patients with cirrhosis limits its common use.31 Rifaximin is a semi-synthetic derivative of rifamycin, has broad-spectrum antimicrobial activity,6 and is characterized by its non-absorbability by the gut.32 Rifaximin remains in high concentrations in its active form in the gut and is excreted in feces without causing significant systemic side effects such as nephrotoxicity.6 Moreover, Rifaximin has been shown in previous studies to be effective at reducing HE parameters in cirrhotics.7-14 However, most reports on this subject have emanated from Europe. It has also been reported that combinatorial rifaximin and lactulose is superior to combined neomycin and lactulose for HE treatment.8 In addition, a recent randomized, controlled study conducted in Spain found that rifaximin and lactitol, a nonabsorbable disaccharide, have similar efficacies for the treatment of acute HE.14
The present study is the first, prospective randomized study to compare the efficacy of rifaximin with that of lactulose for the short-term treatment of HE in Asia. No data is available upon whether ethnic background affects the effectiveness of rifaximin for the treatment of HE. Our study confirms that rifaximin is as effective as lactulose for the treatment of HE in Korean patients. Administration at 1200 mg per day led to an objective and significant improvement in mental state, blood ammonia levels, and HE index. Moreover, no significant difference was found between rifaximin and lactulose in terms of their efficacies. These results suggest that ethnic differences do not significantly affect the efficacy of rifaximin as a HE treatment. In this study, the fact that hepatitis B virus is the predominant (75.9%) cause of HE should be considered. In Western countries, alcoholic abuse remains the most common etiology of liver cirrhosis with HE.18,19 Considering intestinal bacterial overgrowth due to alcohol,20 rifaximin might theoretically be a better choice in alcoholic HE than in viral hepatitisrelated HE. However, our results are similar to those of Western studies concerning the efficacy of rifaximin for the treatment of HE, which suggests that the cause of HE is of secondary importance when considering rifaximin as a therapeutic regimen for HE.
When we analyzed the clinical parameters of patients who showed an improvement with those who did not after rifaximin treatment, several factors were found to be significant by univariate analysis. However, limited patient numbers prevented multivariate analysis. We believe that further study of a larger number of patients would be necessary to identify those factors that determine responsiveness to rifaximin in HE treatment. Interestingly, mean baseline ammonia level and HE index were higher in the improvement group than in the no-improvement group after rifaximin treatment, which suggests that rifaximin can be a first-line choice for the treatment of moderate to severe grade HE.
The identification and correction of factors precipitating HE is of primary concern during the management of HE,29 because the correction of such factors usually results in improvement. Thus patients' precipitating factors should be carefully considered in any future trial. In our study, most patients had an identifiable precipitating factor, and the two treatment groups had reasonably similar precipitating factors profiles. Therefore, we believe that any potential bias caused by precipitating factors was minimal in the present study. Rifaximin was also fairly well tolerated; only one patient experienced abdominal pain attributed to the drug. Renal toxicity and other serious side effects were absent, and no ethnically distinct side effects were observed.
All HE therapeutic trials can be criticized from the perspective of evidence-based medicine.33 Criticisms include the definitions of study endpoints, the treatment of control groups, the proper quantification of therapeutic effects. Sanaka et al. prudently described the difficulties of designing good HE treatment tirals.34 The mental status evaluation system using the portal systemic encephalopathy (PSE) index developed by Conn et al.21 is currently widely used. However, the Food and Drug Administration (FDA) strongly objected to the use of this system and favoured the adoption of a detailed mental status evaluation system for HE.34
Although the present study has a limitation due to its being an open-label study, and may not overcome some of the challenges previously mentioned, it shows that rifaximin is as safe and as effective as lactulose in Korean patients with HE. Rifaximin offers a useful therapeutic option in Asian patients with HE who are unable to tolerate treatment with disaccharides or who have an impaired renal function. Further clinical trials using a new mental state evaluation system, which satisfies the FDA's requirements is required to confirm the efficacy of rifaximin for the treatment of HE.
ACKNOWLEDGEMENTS
The authors thank Ajou Pharmaceutical, Co. Ltd. (Kyunggi-do, Korea) for supplying the rifaximin tablets and lactulose. We also thank Suk Hwa Yoon, RN for technical assistance and data collection.
References
1. Lizardi-Cervera J, Almeda P, Guevara L, Uribe M. Hepatic encephalopathy: a review. Ann Hepatol. 2003; 2:122–130. PMID: 15115963.

2. Riordan SM, Williams R. Treatment of hepatic encephalopathy. N Engl J Med. 1997; 337:473–479. PMID: 9250851.

3. Bosch J, Bruix J, Mas A, Navasa M, Rodes J. Rolling review: the treatment of major complications of cirrhosis. Aliment Pharmacol Ther. 1994; 8:639–657. PMID: 7696453.
4. Clausen MR, Mortensen PB. Lactulose, disaccharides and colonic flora. Clinical consequences. Drugs. 1997; 53:930–942. PMID: 9179525.
5. Weber FL Jr. Lactulose and combination therapy of hepatic encephalopathy: the role of the intestinal microflora. Dig Dis. 1996; 14(Suppl 1):53–63. PMID: 8872452.

6. Gillis JC, Brogden RN. Rifaximin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic potential in conditions mediated by gastrointestinal bacteria. Drugs. 1995; 49:467–484. PMID: 7774516.
7. Testa R, Eftimiadi C, Sukkar GS, De Leo C, Rovida S, Schito GC, et al. A non-absorbable rifamycin for treatment of hepatic encephalopathy. Drugs Exp Clin Res. 1985; 11:387–392. PMID: 3836135.
8. Di Piazza S, Gabriella Filippazzo M, Valenza LM, Morello S, Pastore L, Conti A, et al. Rifaximine versus neomycin in the treatment of portosystemic encephalopathy. Ital J Gastroenterol. 1991; 23:403–407. PMID: 1742538.
9. Pedretti G, Calzetti C, Missale G, Fiaccadori F. Rifaximin versus neomycin on hyperammoniemia in chronic portal systemic encephalopathy of cirrhotics. A double-blind, randomized trial. Ital J Gastroenterol. 1991; 23:175–178. PMID: 1751811.
10. Festi D, Mazzella G, Parini P, Ronchi M, Cipolla A, Orsini M, et al. Treatment of hepatic encephalopathy with non-absorbable antibiotics. Ital J Gastroenterol. 1992; 24:14–16. PMID: 1486194.
11. Bucci L, Palmieri GC. Double-blind, double-dummy comparison between treatment with rifaximin and lactulose in patients with medium to severe degree hepatic encephalopathy. Curr Med Res Opin. 1993; 13:109–118. PMID: 8325041.
12. Miglio F, Valpiani D, Rossellini SR, Ferrieri A. Rifaximin, a non-absorbable rifamycin, for the treatment of hepatic encephalopathy. A double-blind, randomised trial. Curr Med Res Opin. 1997; 13:593–601. PMID: 9327194.

13. Williams R, James OF, Warnes TW, Morgan MY. Evaluation of the efficacy and safety of rifaximin in the treatment of hepatic encephalopathy: a double-blind, randomized, dose-finding multi-centre study. Eur J Gastroenterol Hepatol. 2000; 12:203–208. PMID: 10741936.
14. Mas A, Rodes J, Sunyer L, Rodrigo L, Planas R, Vargas V, et al. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol. 2003; 38:51–58. PMID: 12480560.

15. Reddy KR, Hoofnagle JH, Tong MJ, Lee WM, Pockros P, Heathcote EJ, et al. Racial differences in responses to therapy with interferon in chronic hepatitis C. Consensus Interferon Study Group. Hepatology. 1999; 30:787–793. PMID: 10462387.
16. Nguyen MH, Whittemore AS, Garcia RT, Tawfeek SA, Ning J, Lam S, et al. Role of ethnicity in risk for hepatocellular carcinoma in patients with chronic hepatitis C and cirrhosis. Clin Gastroenterol Hepatol. 2004; 2:820–824. PMID: 15354283.

17. Nair S, Eustace J, Thuluvath PJ. Effect of race on outcome of orthotopic liver transplantation: a cohort study. Lancet. 2002; 359:287–293. PMID: 11830194.

18. Huh K, Choi SY, Whang YS, Lee DS. Prevalence of viral hepatitis markers in Korean patients with hepatocellular carcinoma. J Korean Med Sci. 1998; 13:306–310. PMID: 9681811.

19. Mandayam S, Jamal MM, Morgan TR. Epidemiology of alcoholic liver disease. Semin Liver Dis. 2004; 24:217–232. PMID: 15349801.

20. Morencos FC, de las Heras Castano G, Martin Ramos L, Lopez Arias MJ, Ledesma F, Pons Romero F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci. 1995; 40:1252–1256. PMID: 7781442.
21. Conn HO, Leevy CM, Vlahcevic ZR, Rodgers JB, Maddrey WC, Seeff L, et al. Comparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trial. Gastroenterology. 1977; 72:573–583. PMID: 14049.
22. Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955; 19:393–394. PMID: 13263471.

23. Conn HO. Trailmaking and number-connection tests in the assessment of mental state in portal systemic encephalopathy. Am J Dig Dis. 1977; 22:541–550. PMID: 868833.

24. Elkington SG, Floch MH, Conn HO. Control of chronic portal-systemic encephalopathy by lactulose. Gut. 1969; 10:416. PMID: 4890540.
25. Simmons F, Goldstein H, Boyle JD. A controlled clinical trial of lactulose in hepatic encephalopathy. Gastroenterology. 1970; 59:827–832. PMID: 4922276.

26. Watanabe A, Sakai T, Sato S, Imai F, Ohto M, Arakawa Y, et al. Clinical efficacy of lactulose in cirrhotic patients with and without subclinical hepatic encephalopathy. Hepatology. 1997; 26:1410–1414. PMID: 9397979.

27. Als-Nielsen B, Gluud LL, Gluud C. Non-absorbable disaccharides for hepatic encephalopathy: systematic review of randomised trials. BMJ. 2004; 328:1046–1051. PMID: 15054035.

28. Blei AT, Cordoba J. Hepatic encephalopathy. Am J Gastroenterol. 2001; 96:1968–1976. PMID: 11467622.

29. Kircheis G, Haussinger D. Management of hepatic encephalopathy. J Gastroenterol Hepatol. 2002; 17(Suppl 3):S260–S267. PMID: 12472947.

30. Warren SE, Mitas JA 2nd, Swerdlin AH. Hypernatremia in hepatic failure. JAMA. 1980; 243:1257–1260. PMID: 7359682.

31. Loft S, Sonne J, Dossing M, Andreasen PB. Metronidazole pharmacokinetics in patients with hepatic encephalopathy. Scand J Gastroenterol. 1987; 22:117–123. PMID: 3563404.

32. Descombe JJ, Dubourg D, Picard M, Palazzini E. Pharmacokinetic study of rifaximin after oral administration in healthy volunteers. Int J Clin Pharmacol Res. 1994; 14:51–56. PMID: 7836025.
33. Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy-Definition, nomenclature, diagnosis, and quantification: Final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002; 35:716–721. PMID: 11870389.

34. Sanaka MR, Ong JP, Mullen KD. Challenges of designing hepatic encephalopathy treatment trials. Hepatology. 2003; 38:527–528. PMID: 12903650.

Fig. 1
(A) Changes in grade of blood ammonia level with Rifaximin (solid line) or Lactulose (dotted line) treatment. Grade of blood ammonia was significantly decreased at the end of the treatment with respect to pre-treatment values in both groups (p < 0.01). (B) Changes in HE index with Rifaximin (solid line) and Lactulose (dotted line) treatment. Marked improvement in HE index was noted at the end of the treatment (p < 0.01) compared to pre-treatment values in both groups (p < 0.01).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download