Abstract
The aim of this study was to assess the involvement of multipotential progenitor cells in the pathogenesis of Mooren's ulcer using immunohistochemical staining techniques. Tissue specimens were collected from 3 Mooren's ulcer patients who underwent lamellar keratectomy. Immunohistochemical staining patterns were analyzed using antibodies: CD34, c-kit, STRO-1, CD45RO, VEGF and α-SMA. Strong positive CD34, c-kit and STRO-1 cells were revealed in Mooren's ulcer specimens, especially in the superficial stroma. A few weakly expressed CD34 stroma cells were seen in normal limbal cornea but no immunoreactivity for c-kit and STRO-1 could be found. CD45RO positive T cells were found to have infiltrated in Mooren's ulcer. The immunostaining pattern of VEGF and α-SMA was closely correlated with the degree of expression and the number of CD34 positive cells. Bone marrow-derived multipotential progenitor cells may be involved in the pathogenesis of Mooren's ulcer by synergizing with other factors to amplify autoimmune destructive reactions and to contribute to the regeneration process. Specific therapeutic strategies that target the role of these cells in the disease are warranted.
First described by Bowman in 1849,1 Mooren's ulcer, also known as chronic serpiginous ulcer and ulcer rodens, is a chronic painful corneal ulceration which begins in the peripheral region of the cornea with a steep, undetermined leading edge and spreads both around and centrally to affect the whole cornea.2-4 Due to the rarity of Mooren's ulcer, no controlled and few prospective studies of the treatment of this condition have been conducted. It is always refractory to available therapies, and results in severe visual morbidity.
The pathogenesis of Mooren's ulcer is not well understood. There are substantial evidences suggesting that it is an autoimmune process, with both cell-mediated and humoral components. Reports have described the presence of inflammatory cells5,6 immunoglobulin,7 and increased expression of HLA class II molecules in the cornea and conjunctiva adjacent to the ulcers.8 Cornea-associated antigen (Co-Ag) has also been found in patients' sera.9 The role of co-stimulation in T cell activation has received significant attentions in the last decade and there is little doubt today that T cell function plays a critical role in autoimmunity of Mooren's ulcer.10-12 However, the mechanism which instigates this autoimmune response is still unclear. Currently, there is some evidence supporting the hypothesis that antibodies to cornea antigens are produced primarily in response to tissue damage brought about by the disease, rather than playing a critical role in the initiation of the pathology of the ulcer.13 Other additional factors must be present, even if an association does exist between some of proposed triggers and Mooren's ulcer.
The local manifestation of the systemic disease results in stromal coalescence, and leaves a scarred, vascularized cornea bed. Perforation seldom occurs in Mooren's ulcer because the regeneration process occurs simultaneously. Wound healing processes, including postnatal neovascularization, have been thought to result exclusively from proliferation and migration of preexisting bone marrow multipotential mesenchymal and endothelial progenitor cells.14-16 This finding led us to our research inquiry of better understading the mechanisms of this rare condition from the systemic standpoint. The human hematopoietic progenitor cell antigen CD34 is known to be synthesized and expressed by certain cells of hematopoietic lineage.17,18 Stained tissue for CD34 is usually associated with vascular sprouting during vascularization.19 STRO-1 has been recently shown to differentiate into multiple mesenchymal lineages.20 C-kit is also a hematopoietic progenitor marker and co-expressed with mesenchymal lineages of activated bone marrow stem cells.21 The studies we report herein were carried out by using progenitor cell specific antibodies to determine if they were involved in the pathogenesis of Mooren's ulcer.
Three patients with unilateral Mooren's ulcer having no other pathologic features were recruited from the Department of Ophthalmology, Youngsan Hospital, Choong-Ang University (Seoul, Korea). Clinical descriptions and photographs enabled the diagnosis of Mooren's ulcer to be confirmed in these patients. Informed consent was obtained from all participants for the use of tissue. Tissue specimens were collected from crescent-shaped lamellar keratectomy before lamellar corneoscleral allograft was performed in the involved area.
Immediately after each surgery, some part of the excised tissue was snap frozen after embedment in optimal-cutting-temperature (O.C.T.) compound (Tissue Tek; Miles, Naperville, IL, U.S.A), and the other part was fixed in 10% neutral buffered formalin, and then embedded in paraffin. Several sets of serial 4 to 6µm cryostat sections from each specimen were placed on gelatinized slides, air dried, and then fixed in cold acetone and rinsed in Tris-buffered saline. Paraffin sections were deparaffinized in xylene and descending ethanol series. An immunohistochemical study was carried out using a labeled streptavidin-binotin technique (Histostain-plus Kit; Zymed, South San Francisco, CA, U.S.A.). A panel of primary antibodies for the detection of stem cell surface antigens, including the following: polyclonal goat antihuman CD34, c-kit (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), monoclonal mouse anti-human STRO-1 (DSHB, IA, U.S.A.), monoclonal mouse anti-human α-Smooth muscle actin (α-SMA, NeoMarkers, CA, U.S.A.), monoclonal mouse anti-human CD45RO (DAKO Corporation, CA, U.S.A.), polyclonal rabbit anti-human vascular endothelial growth factor (VEGF, Zymed, South San Francisco, CA, U.S.A.) were used. The specimen was incubated in biotinylated secondary antibody for 1 hour at room temperature, and then incubated for 30 minutes with peroxidase-conjugaed sterptavidin. The presence of peroxidase was revealed by adding substrate-chromogen (3-amino-9-ethycar bazole) solution. Sections were counterstained with hematoxilin.
The number of CD34, VEGF and α-SMA positive cells was counted from three different regions of each sample under 400× magnification.
All 3 patients had a stable and relatively regular ocular surface after lamellar keratectomy, confirmed by topographic examination. Preoperative external photography of 1 selected case, who had significant marginal ulceration with a thinned, vascularized cornea base, is shown in Fig 1. Visual acuity was improved from 20/40 to 20/20 on the initial consultation.
Results from light microscopy of the cornea from Mooren's ulcer patients revealed a focal absence of the corneal epithelium and Bowman's layer. In all the specimens, strong positive immunoreactivity to CD34, c-kit and STRO-1 could be detected in the patients' cornea stroma, especially in the superficial stroma. CD34 was positively expressed by vascular endothelial cells, some stroma cells, and epithelial cells. Some c-kit and STRO-1 positive cells exhibited the typical spindle-shape (Fig. 2A, 2B, 2C). Upregulated activated CD45 positive T cells infiltrated the Mooren's ulcer specimens, especially in the superficial region of stroma (Fig. 3A); Strong positive VEGF staining pattern was shown in vascular endothelial cells, a few epithelial cells, and some scattered cells in stroma (Fig. 3B); α-SMA was positively expressed only in the vessel wall of vascularized stroma (Fig. 3C). Similar immunostaining patterns were found among CD34 (18.50 ± 3.87 positive cells), VEFG (16.75 ± 3.78 positive cells) and α-SMA (7.25 ± 2.21 positive cells) with strong positive expression for small vessels.
Despite accumulating evidence that cell-mediated and humoral immunomechanisms are present in Mooren's ulcer, it is uncertain that they are involved directly in the pathogenesis of the disease. It is possible that they simply accompany corneal destruction that is caused by another mechanism.22 In contrast to some other forms of peripheral ulcerative keratitis, a clinical characteristic of Mooren's ulcer is the involvement of the limbus, where stem cells exist.23,24 With the disease gradually running its course, healing and vascularization follow, which then results in a typically scarred, vascularized cornea that may be thinned to less than half of its original thickness. This is a result of the systemic regeneration process. Because endothelial progenitor cells that contribute to neovascularization are differentiated from CD34 positive cells and mobilized from bone marrow, and scarred cornea mainly consists of differentiated fibroblasts,25 we hypothesize that bone marrow derived multipotential progenitor cells may be involved in co-existing cornea destruction and regeneration during the course of the disease.
VEGF has been identified with vasculogenesis26 and α-SMA stains smooth muscle cells in vessel walls.27 Leukocyte antigen CD34 is the common surface marker for hematopoietic progenitor cells and endothelium.28 Endothelial cell surface receptors that appear early in development are VEGF receptor and CD34.29 The c-kit protooncogene has been identified as a member of the receptor-tyrosine kinase family. When c-kit receptors, which are produced by mesenchymal cells, bind to human stem cell factor (SCF) (also termed c-kit ligand) it will augment the proliferation of primitive bone marrow progenitor cells and differentiated mast cells.30-32 The fibroblast-like stem cells express the c-kit, suggestive of mesenchymal differentiation.21 STRO-1 is a monoclonal antibody that was shown to recognize all the clonogenic mesenchymal stem cells in human bone marrow fibroblast-like cells and to various nonhematopoietic progenitor cells.33,34 Hematopoietic and mesenchymal stem cells detected in Mooren's ulcer cornea in this study may be considered to be evidence of the involvement of bone marrow-derived progenitor cells in the pathogenesis of Mooren's ulcer. Some VEGF and α-SMA positive cells may differentiate from CD34 positive progenitor cells.
The mechanism proposed for the pathogenesis of Mooren's ulcer postulates that circulating immune complexes are deposited in the limbal vessels, resulting in an immune-mediated vasculitis with vessel wall damage and leakage of inflammatory cells and proteins.35,36 Inflammatory cells can release cytokines such as interleukin (IL)-1β, -6,-8, tumor necrosis factor-α (TNF-α) and proteases which destroy corneal stroma.37-39 This immune response will act as a long term signal to stimulate bone marrow quiescent multipotential progenitor cells and chemotaxis to the wounded limbal area to participate in the wound healing and regenerative process. It is conceivable that some factors or combinations of certain cytokines, especially IL-1 and TNF-α which are capable of stimulating keratocytes to release chemotactic factors (e.g.IL-8 and granulocyte colony stimulating factor (GM-CSF)), will activate these localized stem cells and simultaneously activate more neutrophils.40 Following activation, multipotential progenitor cells gain their ability to differentiate into vascular endothelial cells, fibroblasts, and cornea epithelial cells, therefore contributing to cornea vascularization scarring and re-epithelization. In contrast, differentiated fibroblasts, such as capillary endothelial cells, which are capable of producing enzymes that can initiate the degradation of type I collagen, are involved in the destructive process.
Activated progenitor cells can produce numerous cytokines such as IL-6, IL-11, macrophage colony stimulating factor (M-CSF), GM-CSF and SCF.41 These cytokines can further activate more limbal stem cells, neutrophils and macrophages. Activated neutrophils will release neutrophil calgranulin C (CaGC), because the amino acid of cornea-associated antigen (Co-Ag) is found to be identical to that of CaGC.42 It is possible to postulate that progenitor cells indirectly amplify the autoimmune response. In addition, neutrophils may actively participate in the destructive process of Mooren's ulcer by releasing a variety of collagenase.40
In summary, we confirmed the expression of the multipotential progenitor cells and other cooperating factors in Mooren's ulcer. The origin of multipotential stem cells is presumed to be in the bone marrow. Although the detailed mechanisms by which stem cells participate in multi-pathway corneal inflammatory and autoimmune disease require further study, our findings elucidate other molecular mechanisms involved in the process and may help in achieving a more comprehensive view of Mooren's ulcer. It is anticipated that this new knowledge will lead to the development of specific therapeutic strategies that could target critical steps in the pathogenesis of this disease.
Figures and Tables
Fig. 1
Slit lamp photograph of Mooren's ulcer shows advanced corneal ulceration involving corneal center with vascularized corneal base, corneal thinning and adjacent conjuctival inflammation.
Fig. 2
Immunohistochemical staining of the limbal cornea of Mooren's ulcer for antibodies CD34 (A), c-kit (B) and STRO-1 (C). CD34, c-kit and STRO-1 immunoreactivity was strongly positive in the superficial stroma of Mooren's ulcer cornea (Original magnification × 200).
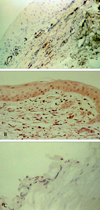
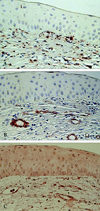
Fig. 3
Patterns of immunostaining of the limbal cornea of Mooren's ulcer with CD45RO (A), VEGF (B) and α-SMA (C). CD45RO positive T cells infiltrated in the superficial stroma (A). Vascular endothelial cells and some stroma cells stained with VEGF (B). α-SMA positive cells located in the vessel wall (Original magnification ×200).
References
1. Robin JB, Schanzlin DJ, Verity SM, Barron BA, Arffa RC, Suarez E, et al. Peripheral corneal disorders. Surv Ophthalmol. 1986. 31:1–36.
2. Brown SI. What is Mooren's ulcer? Trans Ophthalmol Soc UK. 1978. 98:390–392.
3. Wood TO, Kaufman HE. Mooren's ulcer. Am J Ophthalmol. 1971. 71:417–422.
4. Tabbara KF. Mooren's ulcer. Int Ophthalmol Clin. 1986. 26:91–98.
5. Young RD, Watson PG. Light and electron microscopy of corneal melting syndrome (Mooren's ulcer). Br J Ophthalmol. 1982. 66:341–356.
6. Gottsch JD, Liu SH, Minkovitz JB, Goodman DF, Srinivasan M, Stark WJ. Autoimmunity to a cornea-associated stroma antigen in patients with Mooren's ulcer. Invest Ophthalmol Vis Sci. 1995. 36:1541–1547.
7. Eiferman RA, Hyndiuk RA, Hensley GT. Limbal immunopathology of Mooren's ulcer. Ann Ophthalmol. 1978. 10:1203–1206.
8. Lopez JS, Price FW, Whitcup SM, Li Q, de Smet M, Chen CC, et al. Immunopathology of Mooren's ulcer. Arch Ophthalmol. 1991. 109:988–992.
9. Zhao JC, Jin XY. Immunological analysis and treatment of Mooren's ulcer with cyclosporin A. Cornea. 1993. 12:481–488.
10. Romagnani S. Th1 and Th2 in human disese. Clin Immunol Immunopathol. 1996. 80:225–235.
11. Giscombe R, Nityanand S, Lewin N, Grunewald J, Lefvert AK. Expanded T cell populations in patients with Wegener's granulomatosis: characteristic and correlates with disease activity. J Clin Immunol. 1998. 18:404–413.
12. Ludviksson BR, Sneller MC, Chua KS, Talar-Williams C, Langford CA, Ehrhardt RO, et al. Active Wegener's granulomatosis is associated with HLA-DR+ CD4+ T cells exhibiting an unbalanced Th1-type T cell cytokine pattern: reversal with IL-10. J Immunol. 1998. 160:3602–3609.
13. John SL, Morgan K, Tullo AB, Holt PJ. Corneal autoimmunity in patients with peripheral ulcerative keratitis (PUK) in association with rheumatoid arthritis and Wegener's granulomatosis. Eye. 1992. 6:630–636.
14. Risau W. Differentiation of endothelium. FASEB J. 1995. 9:926–933.
15. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997. 275:964–967.
16. Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999. 85:221–228.
17. Boyd AW. Human leukocyte antigens: an update on structure, function and nomenclature. Pathology. 1987. 19:329–337.
18. Watt SM, Karhi K, Gatter K, Furley AJ, Katz FE, Healy LE, et al. Distribution and epitope analysis of the cell membrane glycoprotein (HPCA-1) associated with human progenitor cells. Leukaemia. 1987. 1:417–426.
19. Schlingemann RO, Rietveld FJ, de Waal RM, Bradley NJ, Skene AI, Davies AJ, et al. Leukocyte antigen CD34 is expressed by a subset of cultured endothelial cells and on endothelial abluminal microprocesses in the tumor stroma. Lab Invest. 1990. 62:690–696.
20. Dennis JE, Carbillet JP, Caplan AI, Charbord P. The STRO-1+ marrow cell population is multipotential. Cell Tissues Organs. 2002. 170:73–82.
21. Huss R, Moosmann S. The co-expression of CD117 (c-kit) and osteocalcin in activated bone marrow stem cells in different disease. Br J Haematol. 2002. 118:305–312.
22. Chow CY, Foster CS. Mooren's ulcer. Int Ophthalmol Clin. 1996. 36:1–13.
23. Frangiegh T, Kenyon KR. Brightbill FS, editor. Mooren's ulcer. Corneal surgery: theory, technique and tissue. 1993. ed2. St Louis: Mosby.
24. Robin JB, Dugal R. Kaufman HE, Barron BA, McDonald MB, Saltman SR, editors. Immunologic disorders of the cornea and conjunctiva. The Cornea. 1988. New York: Churchill Livingstones.
25. Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001. 103:2776–2779.
26. Risau W, Flamme I. Vasculogenesis. Anna Rev Cell Biol. 1995. 11:73–91.
27. Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against α-smooth muscle differentiation. J Cell Biol. 1986. 103:2787–2796.
28. Peault BM, Thiery JP, LeDouarin NM. Surface marker for hemopoietic and sndothelial cell lineages that is defined by monoclonal antibody. Pro Natl Acad Sci USA. 1983. 80:2976–2980.
29. Garlanda C, Dejana E. Heterogeneity of endothelial cells: specific markers. Arterioscler Thromb Vasc Biol. 1997. 17:1193–1202.
30. Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990. 63:213–224.
31. Huang E, Nocka K, Beier DR, Chu TY, Buck J, Lahm HW, et al. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990. 63:225–233.
32. Anderson DM, Lyman SD, Baird A, Wignall JM, Eisenman J, Rauch C, et al. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell. 1990. 63:235–243.
33. Gronthos S, Graves SE, Ohta S, Simmons PJ. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994. 84:4164–4173.
34. Simmons PJ, Torok SB. Identification of stroma cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991. 78:55–62.
35. Mondino BJ. Inflammatory disease of the peripheral cornea. Ophthalmology. 1988. 95:463–472.
36. Mondino BJ. Experimental aspects and models of peripheral corneal disease. Int Ophthalmol Clin. 1986. 26:5–14.
37. Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea. 2000. 19:625–643.
38. Gregory JK, Foster CS. Peripheral ulcerative keratitis in the collagen vascular disease. Int Ophthalmol Clin. 1996. 36:21–30.
39. Shiuey Y, Foster CS. Peripheral ulcerative keratitis and collagen vascular disease. Int Ophthalmol Clin. 1998. 38:21–32.
40. Gottsch JD, Li Q, Ashraf F, O'Brien TP, Stark WJ, Liu SH. Cytokine-induced calgranulin C expression in keratocytes. Clin Immunol. 1999. 91:34–40.
41. Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000. 9:841–848.
42. Marti T, Ertmann KD, Gallin MY. Host-parasite interaction in human oncocherciasis: identification and sequence analysis of novel human calgranulin. Biochem Biophys Res Commun. 1996. 221:454–458.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download