Abstract
Pneumomediastinum and subcutaneous emphysema generally occurs following trauma to the esophagus or lung. It also occurs spontaneously in such situations of elevating intrathoracic pressure as asthma, excessive coughing or forceful straining. We report here on the rare case of a man who experienced the signs of pneumomediastinum and subcutaneous emphysema after a prolonged bout of intractable hiccup as the initial presenting symptoms of multiple sclerosis.
Intractable hiccup is an uncommon and incapacitating disturbance that is defined as a hiccup bout lasting more than 48 hours. The various diseases of the gastrointestinal system and central nervous system (CNS) can cause this rare clinical symptom.1-3 Pneumomediastinum and subcutaneous emphysema usually occurs following esophageal or chest trauma. It can also occur spontaneously in association with asthma, excessive coughing or forceful straining during exercise and other situations in which the intra-thoracic pressure is elevated.4,5 Multiple sclerosis has been reported as a rare causative CNS disease for intractable hiccup.6-10 However, pneumomediastinum and subcutaneous emphysema caused by the intractable hiccup in multiple sclerosis has not been reported. We present here a rare case of multiple sclerosis in which pneumomediastinum and subcutaneous emphysema initially developed due to intractable hiccup.
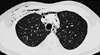
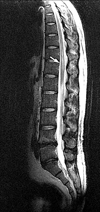
A 26-year-old man was admitted due to his intractable hiccup and subsequent vomiting. The hiccup developed 7 days before his hospital admission and it had persisted all week long. He was good in health before admission. Upon admission he was alert and fully oriented. There was no motor weakness including the facial muscles. The sensory examination revealed hypesthesia on the left face, neck and shoulder areas. The deep tendon reflexes were normoactive and the gag reflex was bilaterally present. Routine laboratory studies showed normal findings except for hypokalemia (2.8 mmol/l) and leukocytosis (13,520/µl). The serum anti-nuclear antibody, anti-double stranded DNA antibody, anti-Ro/La antibody and anti-neutrophil cytoplasmic antibody were negative. Chest x-ray showed subcutaneous emphysema in the right anterior chest wall and neck area, and the chest CT showed pneumomediastinum in the anterior mediastinum as well as subcutaneous emphysema in the right anterior chest wall without there being any evidence of airway or esophageal injury (Fig. 1). There was no evidence of esophageal lesion including any perforation, except for a mild reflux esophagitis that was detected by esophagogasrtoduodenoscopy examination. A CNS lesion was suspected because of the unexplained intractable hiccup and sensory changes in the left face, neck and shoulder areas. Brain MRI (Signa 3.0 T, GE, Milwaukee) performed on the 8th day after symptom onset showed an area of high signal intensity in the left side lower medulla oblongata and upper cervical cord on T2-weighted imaging. These lesions showed mild enhancement after the gadolinium injection (Fig. 2). Cerebrospinal fluid (CSF) examination showed eight white blood cells and no red blood cells. The CSF protein and glucose were 33.5 mg/dL and 72 mg/dL, and no oligoclonal band was found. As we suspected the medullary lesion as an inflammatory process such as acute disseminated encephalomyelitis or the initial manifestation of multiple sclerosis, we treated the patient with intravenous steroid and symptomatically controlled the hiccup with valproic acid and baclofen. We also used prophylactic antibiotics for the prevention of mediastinitis. The hiccup gradually improved and disappeared after the medication. The follow up chest x-ray showed no signs of subcutaneous emphysema at the 5th hospital day. He was discharged at the 10th hospital day. Six weeks after his discharge from hospital, he was readmitted because of voiding disturbance along with numbness and weakness of both his legs. The spine MRI showed a high signal intensity spinal cord lesion at T11-T12 level (Fig. 3). He was diagnosed as having multiple sclerosis, and so he was treated with intravenous steroid, and we started subcutaneous beta interferon injection to prevent recurrence.
Spontaneous pneumomediastinum and subcutaneous emphysema is generally a benign, self-limited condition that usually occurs in situations of excessive elevated intra-thoracic pressure. This elevated intra-thoracic pressure may occur due to bronchial asthma, forceful straining during exercise, inhalation of drugs, childbirth, severe cough or vomiting, as well as other activities associated with the Valsalva maneuver. In this case, there was no history of trauma to the chest or esophagus, and esophagogasrtoduodenoscopy and chest CT revealed no visible lesion of the esophagus, lung and bronchus. Therefore, we concluded that the pneumomediastinum and subcutaneous emphysema were caused by the intractable hiccup that lasted for several days. Spontaneous pneumomediastinum results from the rupture of terminal alveoli into the lung inters titium as a consequence of a pressure gradient existing between the periphery of the lung and the hilum.4 This rupture causes air to dissect a path along the pulmonary vasculature towards the hilum. The gas then travels centrally along the bronchoalveolar trunks, the peribronchial space or within the lymphatics to reach the mediastinum. We can speculate that the intractable hiccup induced the pneumomediastinum by this described mechanism. The hiccup is an abrupt and involuntary contraction of the diaphragm and intercostals muscles; it is of a reflexive origin and is associated with the sudden closure of the glottis. The neural pathway of hiccup has been described as a reflex arc. The vagus nerve, the phrenic nerve and the thoracic sympathetic afferent fibers comprise the afferent limb of this arc, and the efferent limb is the phrenic nerve or the efferent fibers to the respiratory muscles. This neural pathway is also influenced by parts the central nervous pathway such as the respiratory center in the brainstem, the reticular formation of the medulla oblongata, the hypothalamus or the temporal lobe.2-3,7 Hiccup arises when a stimulative disorder occurs in one of the described pathways. Intractable hiccup as the presenting symptom of multiple sclerosis has on rare occasion been reported. In those cases, the plaques detected by MRI have been located in the medulla oblongata, relatively often in the tegmental region,6,9 rarely in the ventral region,8 and very rarely in the cervical cord.7 It has been suggested that the mechanism of intractable hiccup caused by lesions of the central nervous pathway is the result of disinhibition of a primitive reflex, which is normally suppressed by the descending fibers of the CNS.10,11 In our case, the plaque located at the lower medulla oblongata and upper cervical cord may have been the lesion responsible for inducing the intractable hiccup. Although there is no direct causal relationship between multiple sclerosis and pneumomediastinum, this case shows a rare condition in which pneumomediastinum resulted from intractable hiccup, which was the initial presenting manifestation of multiple sclerosis.
Figures and Tables
Fig. 1
Chest CT shows pneumomediastinum and subcutaneous emphysema in the anterior mediastinum and right anterior chest wall.
References
1. Souadjian JV, Cain JC. Intractable hiccup: etiologic factors in 220 cases. Postgrad Med. 1968. 43:72–77.
2. Marsot-Dupuch K, Bousson V, Cabane J, Tubiana JM. Intractable hiccups: the role of cerebral MR in cases without systemic cause. AJNR Am J Neuroradiol. 1995. 16:2093–2100.
3. Nathan MD, Leshner RT, Keller AP Jr. Intractable hiccups (singultus). Laryngoscope. 1980. 90:1612–1618.
4. Koullias GJ, Korkolis DP, Wang XJ, Hammond GL. Current assessment and management of spontaneous pneumomediastinum: experience in 24 adult patients. Eur J Cardiothorac Surg. 2004. 25:852–855.
5. Jougon JB, Ballester M, Delcambre F, Mac Bride T, Dromer CE, Velly JF. Assessment of spontaneous pneumomediastinum: experience with 12 patients. Ann Thorac Surg. 2003. 75:1711–1714.
6. Park KW, Cheon SM, Hong SK, Choi SH, Cha JK. A case of intractable hiccups as presenting symptom of multiple sclerosis. J Korean Neurol Assoc. 2002. 20:195–198.
7. Funakawa I, Terao A. Intractable hiccups and syncope in multiple sclerosis. Acta Neurol Scand. 1998. 98:136–139.
8. Chang YY, Wu HS, Tsai TC, Liu JS. Intractable hiccup due to multiple sclerosis: MR imaging of medullary plaque. Can J Neurol Sci. 1994. 21:271–272.
9. Funakawa I, Hara K, Yasuda T, Terao A. Intractable hiccups and sleep apnea syndrome in multiple sclerosis: report of two cases. Acta Neurol Scand. 1993. 88:401–405.
10. McFarling DA, Susac JO. Hoquet diabolique: intractable hiccups as a manifestation of multiple sclerosis. Neurology. 1979. 29:797–801.
11. Al Deeb SM, Sharif H, Al Moutaery K, Biary N. Intractable hiccup induced by brain stem lesion. J Neurol Sci. 1991. 103:144–150.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download