Abstract
Phosphodiesterase (PDE) 4 inhibitors have been shown to induce the cAMP-mediated signaling pathway by inhibiting cAMP hydrolysis. This study investigated the effect of a PDE4 inhibitor on the expression of the inducible cAMP early repressor (ICER), which is an endogenous inhibitor of CRE-mediated transcription, in osteoblastic cells. RT-PCR analysis revealed that rolipram, a PDE4 inhibitor, stimulates the ICER mRNA in a dose dependent manner. The induction of ICER mRNA expression by rolipram was suppressed by the inhibitors of protein kinase A (PKA) and p38 MAPK, suggesting the involvement of PKA and p38 MAPK activation in ICER expression by rolipram. It was previously shown that rolipram induced the expression of TNF-related activation-induced cytokine (TRANCE, also known as RANKL, ODF, or OPGL) in osteoblasts. This paper provides evidences that a transcriptional repressor like ICER might modulate TRANCE mRNA expression by rolipram in osteoblasts.
Osteoporosis and other diseases involving bone loss are a major public health problem. Despite the recent successes with drugs that inhibit bone resorption, notably bisphosphonates, there is a clear therapeutic need for bone anabolic molecules, particularly in patients who have already suffered substantial bone loss.
Parathyroid hormone (PTH) and prostaglandin E2 (PGE2) stimulate bone formation in experimental animals and humans.1-3 Several studies suggest that cyclic AMP (cAMP), which initiates protein kinase A (PKA) signaling, mediates the anabolic effects of these two molecules.3,4 Many cAMP-responsive genes have been identified in PTH-treated osteoblasts, including collagenase,5 c-fos,6 type I collagen,7 interleukin-6,8 cycloxygenase-2 (cox-2),9 TNF-related activation-induced cytokine (TRANCE, also known as RANKL, ODF, or OPGL),10 and inducible cAMP early repressor (ICER).11
ICER is a member of the cAMP response element binding protein (CREB) and CRE modulator (CREM) family of transcription factors, which bind to CREs.12 The ICER is generated in an inducible manner when an internal promoter of the CREM gene, containing CRE sites, is stimulated by increased cAMP levels.12 Because the ICER consists of only a DNA-binding domain identical to the one in the CREM and lacks the transactivation domain, the ICER serves as a dominant-negative of CREM/CREB-mediated transcription.12
Intracellular cAMP is generated by adenylate cyclase from adenosine triphosphate (ATP) as a substrate, whereas cAMP-specific phosphodiesterases (PDEs) catalyze the hydrolysis of cAMP to 5'-AMP.13,14 Therefore, the intracellular cAMP gradients are governed by a balance between its generation by adenylate cyclase and degradation by the PDEs.
The PDE family consists of 11 isozymes ranging from PDE1 to 11. Those isozymes involved in the degradation of cAMP are PDE1, 2, 3, 4, 7, 8, 10, and 11, with some of these PDE isozymes being further classified into subtypes.14 Rolipram, a PDE4 specific inhibitor, has recently been demonstrated to increase the bone mass mainly by promoting bone formation in normal mice.15 Furthermore, PDE4 inhibitors have been shown to have therapeutic effects in different experimental osteopenia models.16,17 Although it has been hypothesized that PDE4 inhibitors can mimic the anabolic effects of PTH and PGE2 on the bone, little is known about the precise mechanism by which the PDE4 inhibitors regulate the expression of the osteoblastic genes.
In this study, rolipram was shown to induce ICER mRNA expression in mouse osteoblastic cells. It was found that rolipram-dependent ICER mRNA expression was mediated possibly by the PKA and p38 mitogen-activated protein kinase (MAPK) pathway, with little contribution from the extracellular signal-regulated kinase (ERK) MAPK pathway. It was also suggested that ICER might play an important modulatory role in the rolipram-mediated regulation of TRANCE, which is an essential molecule for osteoclastogenesis,18-20 in osteoblasts.
H89, PD98059 and SB203580 were obtained from Calbiochem (San Diego, CA). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Primary calvarial osteoblasts were isolated from the calvariae of neonatal ddY mice (Japan SLC Inc., Shizuoka, Japan) by a conventional method using 0.1% collagenase and 0.2% dispase. UAMS-32, which is an osteoblastic/stromal cell line, was a kind gift from Prof. Masamichi Takami (Showa University, Tokyo, Japan). All the cells were cultured in α-MEM/10% FBS at 37℃ and 5% CO2.
Total RNA (1 µg) was reverse-transcribed using Superscript II (Invitrogen, CA, USA) according to the manufacturer's protocols. Aliquots of the obtained cDNA pool were subjected to PCR amplification with Go Taq DNA polymerase (Promega Co., WI, USA). The primers for ICER and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) used in this study are as follows: ICER, 5'-gatactggagatgaaactga-3' (forward), 5'-ctttctcatacagttcacag-3' (reverse); and GAPDH, 5'-gaaggtcggtgtgaacggatttggc-3' (forward), 5'-catgtaggccatgaggtccaccac-3' (reverse). The PCR program is as follows: 40 (ICER) or 28 (GAPDH) cycles, after an initial denaturation step at 94℃ for 3 minutes, then denaturation at 94℃ for 30 seconds, annealing at 48℃ (ICER) or 52℃ (GAPDH) for 45 seconds, and extension at 72℃ for 60 seconds, with a final extension at 72℃ for 10 minutes.
Total protein extracts were isolated from the rolipram-treated UAMS-32 cells. After separation in SDS-PAGE, the proteins were transferred onto Immobilon-P membranes (Millipore, Bedford, MA). The membranes were blocked with 5% nonfat-milk in TBS-T (150 mM NaCl, 20 mM Tris, pH 7.4, 0.1% Tween 20), and then immunostained with either the anti-phospho p38 antibody (1:1000) or the anti-p38 antibody (1:1000) followed by secondary horseradish peroxidase-conjugated antibody (1:5000, Cell Signaling Technology). The membranes were developed using an enhanced chemiluminescence detection kit (Amersham Biosciences, Bukinghamshire, UK).
Cells in 60-mm diameter dishes were cultured with the agents for the indicated periods, and subjected to total RNA isolation using Trizol reagent (Invitrogen, CA, USA) according to the manufacturer's protocol. The total RNA (20 µg) was electrophoresed in 1.2% agarose-formaldehyde gels, transferred to nylon membrane filters (Hybond N+, Amersham Biosciences, Bukinghamshire, UK), and hybridized with the 32P-labeled cDNA probes. The cDNAs encoding TRANCE and GAPDH cloned by polymerase chain reaction (PCR) were used as the probes. After the final wash, the membrane was exposed to an X-ray film (BioMax, Kodak, Rochester, NY) at -70℃.
The effect of rolipram, a selective PDE4 inhibitor, on ICER mRNA expression was examined using RT-PCR in osteoblastic cells. Although there are four isozymes in the ICER family (ICER-I, ICER-Iγ, ICER-II, and ICER IIγ), the function of each isoform identified thus far is indistinguishable.12 Rolipram induced mRNA expression of ICER-I (700 bp) and ICER-II (262 bp) in UAMS-32 cells as early as 1 hr, which persisted for up to 9 hrs (Fig. 1A). In order to determine the dose-dependence of rolipram-induced ICER mRNA expression, the UAMS-32 cells were treated with various concentrations of rolipram for 3 hrs. Fig. 1B shows that 0.01-10 µM rolipram stimulated the ICER in a dose-dependent manner. These results demonstrate that rolipram strongly induces ICER mRNA expression in osteoblastic cells.
In order to identify the signaling pathways used by rolipram to induce ICER expression, the roles of the PKA-, ERK-, and p38 MAPK signaling cascades in ICER expression were examined. The cAMP-PKA pathway is essential for PTH-dependent ICER expression in osteoblasts.21 Consistent with this, pretreatment of UAMS-32 cells with H89, a PKA inhibitor, decreased the ICER mRNA expression levels induced by rolipram (Fig. 2A). Furthermore, SB203580, a p38 MAPK inhibitor, also decreased the ICER mRNA expression levels induced by rolipram. However, a MAPK/ERK kinase (MEK) inhibitor, PD98059, did not affect the ICER mRNA levels induced by rolipram (Fig. 2A). Immunoblot analysis with a phospho-p38-specific antibody confirmed that rolipram stimulates the rapid phosphorylation of p38 with its maximum effect at 15 min (Fig. 2B). This suggests that rolipram regulates ICER mRNA expression in osteoblastic cells possibly via the activation of the PKA and p38 MAPK signaling pathways, but not via the activation of the ERK MAPK pathway.
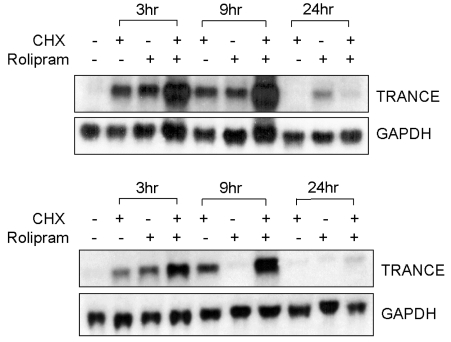
It was previously reported that rolipram stimulates TRANCE mRNA expression in osteoblastic cells.22 In order to determine if intermediate gene expression is essential for the effects of rolipram on TRANCE expression, the UAMS-32 cells were treated with rolipram for 3, 9, 24 hrs in the presence or absence of the protein synthesis inhibitor, cycloheximide. Cycloheximide alone stimulated the TRANCE mRNA levels at 3 and 9 hrs but did not block the additional stimulatory action of rolipram in the UAMS-32 cells (Fig. 3A). This indicates that rolipram stimulates TRANCE mRNA expression in osteoblastic cells directly. The expression level of TRANCE mRNA by rolipram decreased at 9 hrs and returned almost to the baseline by 24 hrs (Fig. 3A). However, pretreatment of cycloheximide caused a continuing increase in the TRANCE mRNA expression level at 9 hrs, suggesting the possible involvement of a transcriptional repressor such as ICER on TRANCE expression in osteoblastic cells. Primary mouse calvarial cells were used in order to exclude the possibility that the effect of cycloheximide on rolipram-induced TRANCE expression is unique in UAMS-32 cells. As shown in Fig. 3B, similar results were also obtained in primary mouse calvarial cells.
This study showd that rolipram, a PDE4 inhibitor, rapidly induces ICER mRNA in UAMS-32 osteoblastic cells (Fig. 1). A similar induction of ICER mRNA by cAMP was reported in other cells and tissues including pituitary corticotrophs,23 PC12 cells,12 mouse calvaria11 and rat thyroid gland.24 The stimulation of the PKA pathway has been suggested to be a mediator of cAMP-induced ICER mRNA expression in many experimental models.12,24,25 In agreement with this, rolipram was found to induce ICER mRNA expression in osteoblastic cells via the PKA signaling pathway (Fig. 2A).
Interestingly, nerve growth factor (NGF) was demonstrated to induce ICER in adrenal PC12 cells via the ras-MAP kinase pathway.26 It was shown that the activation of p38 MAPK, as well as PKA, possibly mediates ICER mRNA induction by rolipram in osteoblastic cells (Fig. 2). These results suggest that ICER expression may not be exclusively coupled to the cAMP-PKA pathway.
ICER is believed to be a repressor of cAMPinduced transcription. For example, in the pineal gland ICER has been reported to be involved in the repression of melatonin synthesis during the course of the normal circadian rhythm.25 ICER inhibits the PKA-stimulated transcriptional activity of tyrosine hydroxylase in PC12 cells.27 ICER has also been shown to play a regulatory role in PTH-stimulated COX-2 transcription in osteoblastic cells.9 The mechanism by which ICER inhibits the cAMP-inducible genes typically includes binding to a variety of cAMP response elements (CREs) or CRE-like sequences in the promoters of target genes that are binding sites for the activator forms of the CRE-binding protein (CREB) and/or the related factors of the CREM.12 Because the ICER lacks the CREM transactivation domain,12 which is similar in sequence to that of CREB, the binding of ICER to a CRE does not allow the recruitment of either CREB or the CREB-binding protein (CBP/p300) to activate transcription.
The activation of the cAMP-PKA pathway induces the expression of TRANCE in osteoblasts, which triggers osteoclast differentiation.28,29 Recently, cAMP-stimulated TRANCE expression was demonstrated to involve CREB-mediated transcription.30 The attenuation of rolipram-induced TRANCE mRNA expression was shown to require the de novo protein synthesis in osteoblasts (Fig. 3), suggesting the possible role of a repressor protein such as ICER. It has been suggested that the rapid activation of ICER could serve as a protective mechanism by which the cell limits mRNA accumulation of the CRE-containing genes, and that this regulation subverts the pathological consequences of the chronic stimulation of activation of PKA.23 Therefore, the enhanced expression of TRANCE by the activation of PKA would be determined by a complex balance of activation by CREB and repression by ICER, providing a system for the fine modulation of gene expression. Although no direct evidence on the functional ability of ICER to negatively regulate TRANCE mRNA expression is currently available, it is likely that future studies will reveal the additional roles of the ICER in the down-regulation of TRANCE mRNA expression.
References
1. Jee WS, Ma YF. The in vivo anabolic actions of prostaglandins in bone. Bone. 1997; 21:297–304. PMID: 9315332.
2. Lim SK, Won YJ, Park DH, Shin DH, Yook JI, Lee HC, et al. Intermittent parathyroid hormone treatment can promote linear growth in the ovariectomized growing rat. Yonsei Med J. 1999; 40:166–172. PMID: 10333721.

3. Whitfield JF, Morley P, Willick GE. The bone-building action of the parathyroid hormone: implications for the treatment of osteoporosis. Drugs Aging. 1999; 15:117–129. PMID: 10495071.
4. Scutt A, Zeschnigk M, Bertram P. PGE2 induces the transition from non-adherent to adherent bone marrow mesenchymal precursor cells via a cAMP/EP2-mediated mechanism. Prostaglandins. 1995; 49:383–395. PMID: 7480806.

5. Scott DK, Brakenhoff KD, Clohisy JC, Quinn CO, Partridge NC. Parathyroid hormone induces transcription of collagenase in rat osteoblastic cells by a mechanism using cyclic adenosine 3',5'-monophosphate and requiring protein synthesis. Mol Endocrinol. 1992; 6:2153–2159. PMID: 1337147.

6. Clohisy JC, Scott DK, Brakenhoff KD, Quinn CO, Partridge NC. Parathyroid hormone induces c-fos and c-jun messenger RNA in rat osteoblastic cells. Mol Endocrinol. 1992; 6:1834–1842. PMID: 1480173.

7. Kream BE, LaFrancis D, Petersen DN, Woody C, Clark S, Rowe DW, et al. Parathyroid hormone represses alpha 1(I) collagen promoter activity in cultured calvariae from neonatal transgenic mice. Mol Endocrinol. 1993; 7:399–408. PMID: 8483479.

8. Greenfield EM, Gornik SA, Horowitz MC, Donahue HJ, Shaw SM. Regulation of cytokine expression in osteoblasts by parathyroid hormone: rapid stimulation of interleukin-6 and leukemia inhibitory factor mRNA. J Bone Miner Res. 1993; 8:1163–1171. PMID: 8256653.

9. Tetradis S, Pilbeam CC, Liu Y, Herschman HR, Kream BE. Parathyroid hormone increases prostaglandin G/H synthase-2 transcription by a cyclic adenosine 3',5'-monophosphate-mediated pathway in murine osteoblastic MC3T3-E1 cells. Endocrinology. 1997; 138:3594–3600. PMID: 9275040.
10. Lee SK, Lorenzo JA. Regulation of receptor activator of nuclear factor-kappa B ligand and osteoprotegerin mRNA expression by parathyroid hormone is predominantly mediated by the protein kinase a pathway in murine bone marrow cultures. Bone. 2002; 31:252–259. PMID: 12110442.
11. Tetradis S, Nervina JM, Nemoto K, Kream BE. Parathyroid hormone induces expression of the inducible cAMP early repressor in osteoblastic MC3T3-E1 cells and mouse calvariae. J Bone Miner Res. 1998; 13:1846–1851. PMID: 9844102.

12. Molina CA, Foulkes NS, Lalli E, Sassone-Corsi P. Inducibility and negative autoregulation of CREM: an alternative promoter directs the expression of ICER, an early response repressor. Cell. 1993; 75:875–886. PMID: 8252624.

13. Antoni FA. Molecular diversity of cyclic AMP signalling. Front Neuroendocrinol. 2000; 21:103–132. PMID: 10764527.

14. Essayan DM. Cyclic nucleotide phosphodiesterases. J Allergy Clin Immunol. 2001; 108:671–680. PMID: 11692087.

15. Kinoshita T, Kobayashi S, Ebara S, Yoshimura Y, Horiuchi H, Tsutsumimoto T, et al. Phosphodiesterase inhibitors, pentoxifylline and rolipram, increase bone mass mainly by promoting bone formation in normal mice. Bone. 2000; 27:811–817. PMID: 11113392.

16. Waki Y, Horita T, Miyamoto K, Ohya K, Kasugai S. Effects of XT-44, a phosphodiesterase 4 inhibitor, in osteoblastgenesis and osteoclastgenesis in culture and its therapeutic effects in rat osteopenia models. Jpn J Pharmacol. 1999; 79:477–483. PMID: 10361888.
17. Miyamoto K, Waki Y, Horita T, Kasugai S, Ohya K. Reduction of bone loss by denbufylline, an inhibitor of phosphodiesterase 4. Biochem Pharmacol. 1997; 54:613–617. PMID: 9337078.

18. Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, et al. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem. 1997; 272:25190–25194. PMID: 9312132.

19. Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998; 95:3597–3602. PMID: 9520411.

20. Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998; 93:165–176. PMID: 9568710.

21. Nervina JM, Tetradis S, Huang YF, Harrison D, Molina C, Kream BE. Expression of inducible cAMP early repressor is coupled to the cAMP-protein kinase A signaling pathway in osteoblasts. Bone. 2003; 32:483–490. PMID: 12753864.

22. Cho ES, Yu JH, Kim MS, Yim M. Rolipram, a phosphodiesterase 4 inhibitor, stimulates osteoclast formation by inducing TRANCE expression in mouse calvarial cells. Arch Pharm Res. 2004; 27:in press.

23. Lamas M, Sassone-Corsi P. The dynamics of the transcriptional response to cyclic adenosine 3',5'-monophosphate: recurrent inducibility and refractory phase. Mol Endocrinol. 1997; 11:1415–1424. PMID: 9280057.

24. Lalli E, Sassone-Corsi P. Thyroid-stimulating hormone (TSH)-directed induction of the CREM gene in the thyroid gland participates in the long-term desensitization of the TSH receptor. Proc Natl Acad Sci USA. 1995; 92:9633–9637. PMID: 7568187.

25. Stehle JH, Foulkes NS, Molina CA, Simonneaux V, Pevet P, Sassone-Corsi P. Adrenergic signals direct rhythmic expression of transcriptional repressor CREM in the pineal gland. Nature. 1993; 365:314–320. PMID: 8397338.
26. Monaco L, Sassone-Corsi P. Cross-talk in signal transduction: Ras-dependent induction of cAMP-responsive transcriptional repressor ICER by nerve growth factor. Oncogene. 1997; 15:2493–2500. PMID: 9395245.

27. Tinti C, Conti B, Cubells JF, Kim KS, Baker H, Joh TH. Inducible cAMP early repressor can modulate tyrosine hydroxylase gene expression after stimulation of cAMP synthesis. J Biol Chem. 1996; 271:25375–25381. PMID: 8810303.

28. Kondo H, Guo J, Bringhurst FR. Cyclic adenosine monophosphate/protein kinase A mediates parathyroid hormone/parathyroid hormone-related protein receptor regulation of osteoclastogenesis and expression of RANKL and osteoprotegerin mRNAs by marrow stromal cells. J Bone Miner Res. 2002; 17:1667–1679. PMID: 12211438.

29. Kaji H, Sugimoto T, Kanatani M, Fukase M, Kumegawa M, Chihara K. Prostaglandin E2 stimulates osteoclast-like cell formation and bone-resorbing activity via osteoblasts: role of cAMP-dependent protein kinase. J Bone Miner Res. 1996; 11:62–71. PMID: 8770698.

30. Fu Q, Jilka RL, Manolagas SC, O'Brien CA. Parathyroid hormone stimulates receptor activator of NFkappa B ligand and inhibits osteoprotegerin expression via protein kinase A activation of cAMP-response element-binding protein. J Biol Chem. 2002; 277:48868–48875. PMID: 12364326.
Fig. 1
Effect of rolipram, a PDE4 inhibitor, on ICER mRNA expression in UAMS-32 cells. A. The cells were treated with 5 µM rolipram for the indicated times, or B. with the indicated concentrations of rolipram for 3 hrs. The total RNA was prepared and reverse transcribed in the presence (+RT) or absence (-RT) of reverse transcriptase, and the cDNA was amplified using specific primers designed for the genes of ICER-I (700 bp) and -II (262 bp). M=0.1 kb DNA marker.
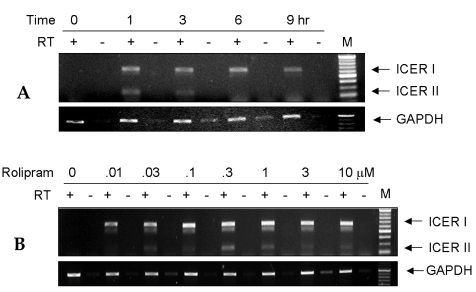
Fig. 2
The involvement of signal transduction pathways in ICER mRNA expression by rolipram. A. The UAMS-32 cells were preincubated in the presence or absence of 30µ M of H89, 50 µM of PD98059, or 50 µM of SB203580 (inhibitors of PKA, ERK, and p38 MAPK, respectively) for 30 min, and then treated with 5µM rolipram for 3 hrs. Total RNA was extracted from the cells and the expression of the ICER was examined by RT-PCR analysis. M=0.1kb DNA marker. B. The UAMS-32 cells were incubated with 5µM rolipram for the indicated times. The total cellular protein was analyzed by immunoblotting with the antibodies for phospho-p38 after which the membranes was stripped and incubated with the anti-p38 antibodies.
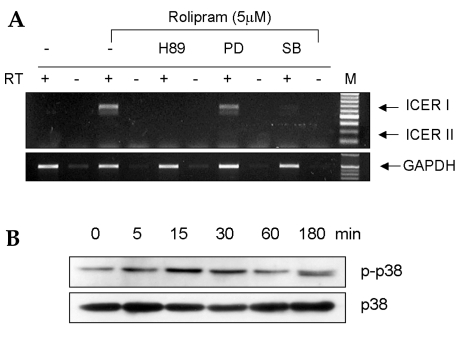
Fig. 3
The attenuation of rolipram-induced TRANCE mRNA expression requires the transcriptional repressor in osteoblasts. A. UAMS-32 cells were treated with 5 µM rolipram, 1 µg/ml cycloheximide (CHX) or rolipram and CHX (the CHX was added 1hr before the rolipram). The total RNA was extracted from the cells at the indicated times. Total RNA was analyzed by Northern blot using probes for TRANCE and GAPDH. B. primary mouse calvarial cells were treated in a similar manner shown in A.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download