Abstract
An endovascular intervention is a feasible alternative to the technically challenging conventional surgery for the treatment of traumatic vertebral arterial lesions. This report describes a rare case involving a 22-year-old patient with a traumatic vertebral arterial pseudoaneurysm and multiple arteriovenous fistulas which were successfully sealed using the endovascular stent-graft technique.
Vertebral artery pseudoaneurysms and vertebrojugular arteriovenous (A-V) fistulas are uncommon lesions and are usually caused by iatrogenic trauma (1). Conventionally, such lesions are treated by surgery, which is quite invasive. However, recently alternative endovascular techniques are introduced, which allow for the selective occlusion of the vascular injury. Both methods do however often require sacrificing the vertebral artery (2). Another alternative to the endovascular approach is the use of covered stent grafts, which maintain vessel patency. However the stents are not specifically designed for neurointerventional use (3). Herein, we report a case of a traumatic pseudoaneurysm with multiple vertebrojugular A-V fistulas which were successfully treated with a small-caliber stent-graft.
A 22-year-old male patient complaining of a pulsatile mass on the left side of his neck was referred to our hospital. The patient had received a knife wound while serving in the military a year prior. The pulsatile cystic mass was consistent with a 3 cm pseudoaneurysm on a color Doppler sonography originating from the cervical segment of the left vertebral artery with a to-and-fro type flow on the pulsed mode. Other imaging studies, including a computerized tomography, magnetic resonance imaging and magnetic resonance angiography were performed which revealed, in consensus, a vascular mass originating from the left vertebral artery which caused a compression on the hypopharynx.
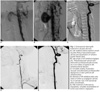
The patient was thoroughly informed of the lesion as well as the outcomes and possible complications of the various available treatment methods. After informed consent was obtained for the stent-graft procedure and other endovascular occlusive treatments, a digital subtraction angiography (DSA) (Multistar Plus/T.O.P., Siemens AG, Forchheim, Germany) was performed using Five French catheters (Weinberg, Digiflex, Boston Scientific Corporation, Watertown, MA), which were introduced into the vertebral arteries via the femoral approach. The selective views of the vertebral arteries were taken by injecting 6 ml of nonionic contrast agent (Iomeron 400 mgI/ml, Bracco SpA, Milan, Italy). The angiography confirmed a 3 cm pseudoaneurysm at the cervical segment of the vertebral artery in addition to multiple arterivenous fistulas (Figs. 1A, B). The right vertebral artery was patent with adequate basilar artery flow in addition to the cross-filling of the left vertebral artery from the right vertebral artery with retrograde opacification of the fistulas. The markings on the catheter served for sizing the diameter of the vertebral artery as well as the careful measure of the pseudoaneurysm. In addition, special care was taken to identify the exact origin and level of the arteriovenous fistulas via multiple injections and meticulous analysis of the angiographic sequences.
By gentle methodical probing of the left vertebral artery, a 7-Fr guiding catheter (Jomed GmBH, Coronary Guiding Catheter, JR 4.0 SH, Germany) was put in. Next, a 300-cm long, by 0.014 diameter stiff guide wire (Urolix, Urtech, GmbH, Germany) was advanced into the distal portion of the vertebral artery. In addition, a polytetrafluoroethylene (PTFE)-covered stent-graft (19-mm long by 4.0 mm diameter, Jostent Coronary Stent-graft Supreme System; Jomed GmBH, Rangendingen, Germany) was advanced until both the pseudoaneurysm ostium and A-V fistula ostia were covered. The oversize rate was approximately 0.4 mm, as a 4 mm stent-graft was used in a 3.6 mm vertebral artery. With a few remaining fistulas apparent on the angiogram caudal to the initial stent graft, a second PTFE-covered stent-graft was deployed with a slight inferior overlap with the first PTFE-covered stent-graft. After each deployment, the balloon expansion of each stent was performed (5.0 mm diameter - 20 mm length Smash Peripheral PTA Balloon Catheter, Boston Scientific Corporation Ireland LTD., Galway, Ireland) up to 18 atm. During deployment of the stent-graft, the patient felt pain on the left side of his neck, probably due to stretching of the vessel. This pain was relieved in a few minutes after deployment. The angiograms revealed that the pseudoaneurysm and all of the arteriovenous fistulas were completely sealed and that the distal flow was improved (Fig. 1C). The recovery was uneventful and the patient was discharged with a prescription of 75 mg clopidogrel per day. The patient was informed of the possible hematologic complications of this medication. Despite the disclaimer, the patient remained on the medication based on agreeable patient compliance and close surveillance of the hematologic parameters.
After 18 months, a follow-up angiogram showed stenosis within the vertebral stent (Fig. 1D). A balloon angioplasty was undertaken over a guide wire (0.014 in by 180-cm guide wire, Wizdom, Cordis Corporation, Miami, FL) using a balloon catheter (4.0 mm diameter - 20.0 mm length Orix Coronary Dilatation Catheter, Modular Stent Systems, Inc., Anaheim, CA). After completing the procedure, better luminal filling was observed on the angiogram (Fig. 1E).
The causes of this rare vertebral arterial injury include atrogenic trauma during catheterization, angiography, nerve blocks, spine surgery, chiropractic manipulation, radiation therapy, penetrating trauma and vasculitis. The possible mechanisms behind this form of arterial include direct arterial puncture, laceration and dissection. The consequences of this injury include stenosis, thrombosis, hemorrhage, pseudoaneurysms and A-V fistulas (1).
Another unlikely and rare source of vertebral arterial injury (due to the deep posterior location and intraforaminal long course of the artery) includes the penetrating trauma of the vertebral artery (1, 4, 5). It is unusual to encounter a traumatic vertebral A-V fistula considering that traumatic arteriovenous fistulas account for approximately 7% of combat related traumatic arterial injuries (with only 25% involving the head and neck region). Moreover, civilian incidences are even rarer (4, 6). Consistent with our case, vertebral traumatic fistulas most commonly involve the second portion of the vertebral artery located within the foramen of the 2nd and 6th vertebra (4, 7). Although a vertebrojugular arteriovenous fistula and a pseudoaneurysm are different patologies, in the setting of penetrating injury, it is not uncommon for these two entities to coexist (1).
Treatment of arteriovenous fistulas often require direct closure of the fistula or occlusion of the vertebral artery, above and below the level of the fistula. The methods used to achieve this include surgical ligation, embolization by detachable balloons, coils or other agents (2, 4, 7). Pseudoaneurysms involving the extracranial vertebral artery either resolve spontaneously or are treated by occluding the parent artery (surgically or endovascularly). In selected cases, embolization of the pseudoaneurysm lumen with embolic material (often coils), using microcatheters represents a possible alternative treatment method (8).
Both the surgical and endovascular procedures involve the occlusion of the arterial lumen, which is the main disadvantage of these methods. For the patients whose contralateral vertebral artery is hypoplastic or affected by atherosclerotic changes, it is often impossible to perform an arterial occlusion due to lack of an adequate intracranial collateral circulation and consequent ischemia (8, 9). In addition, vertebral arteries may be congenitally absent in 3.1% of cases on the left side and 1.8% of cases on the right side (10). Despite adequate collateral flow, upto 8% or higher complication rates are reported upon ligation of a vertebral artery (10). Moreover; in the surgical series relating vertebral injury during spinal surgery, a 12% mortality rate is observed in acute surgical ligation of a vertebral artery (11, 12).
Surgical methods are often complex and require a large area to be exposed, which is not easily accessible (i.e., the base of the skull which is associated with significant risk of complications such as phrenic nerve or Batson venous plexus injury) (4, 13). On the other hand, the endovascular technique involves less pain and disability, as well as a more rapid recovery time compared to the standard surgical technique (14).
The treatment modality we selected for the very rare combination of traumatic pseudoaneurysm and multiple arteriovenous fistulas as observed in our patient was to seal the pseudoaneurysm and occlude all the fistulas while preserving the parent artery. Given that the right vertebral artery provided sufficient flow for posterior circulation, occlusion of the left vertebral artery was also considered. But with so many fistulas, the possibility of a distal vertebral artery retrograde steal in too proximal an occlusion, or cross filling from the right vertebral artery in too distal occlusion were brought up (4).
In our patient, the use of a commercially available stent graft, which was not specifically designed for neuroendovascular use, represented the main drawback of the alternative procedure. The problem with these stents is that they are expensive, rigid, lack flexibility and require high deployment pressure and size mismatch (3).
A literature search was performed on previous studies which used a stent graft in traumatic vertebral artery lesions (using Pub-Med as a source). We found that 11 cases of vertebral A-V fistulas (2, 3, 13, 15-22), five cases of pseudoaneurysms (3, 9, 10, 19, 23) and one dissection (5) were treated with stent grafts. PTFE-covered coronary stents (Jomed) (3, 9, 10, 13, 15, 22) were used in the majority of the cases, but Wallstent (2, 18), Corvita (5) and Hemobahn (20) stents were also reported. The outcomes and patency rates were favorable in all cases. Short and middle term follow-up results were reported in a majority of cases ranging from nine (2) to 16 months (13). The shortest available follow-up time was two months (10), whereas the longest was five years (3).
Although positive outcomes have been reported in both our study and others, the procedure is technically challenging and the delicate vertebral artery, with its small size and long course, is prone to iatrogenic injuries, especially when involving dissection and thrombosis (2, 3, 9, 14). In our case, the stent-grafts were adapted to the vessel wall and the sealing occurred immediately after the balloon dilatation. No procedural and technical problems were encountered.
Fistulas and aneurysms may recur and stenosis or thrombosis of the stent may occur. Therefore, a follow-up is recommended for this procedure. Antiplatelet-antiaggregation therapy is needed to avoid restenosis and thrombosis of the stent grafts which may pose a problem in patients who should avoid such a therapy. The use of PTFE grafts is reportedly advantageous in regards to restenosis and intimal hyperplasia (3). Despite the use of both a PTFE stent and antiaggregation regimen, our patient developed restenosis after 18 months, which was however successfully treated by balloon angioplasty.
In conclusion, when decision making is based on lesion and patient characteristics or other selected cases, the endovascular graft stenting technique appears to be a feasible and effective alternative to surgical procedures for the treatment of traumatic pseudoaneurysms or arteriovenous fistulas in vertebral arteries (1-3, 5, 10, 13).
Figures and Tables
Fig. 1
Endovascular stent-graft treatment in 22-year-old man.
A. Left vertebral arterial injection revealing early filling of pseudoaneurysm (arrow) and opacification of vertebrojugular fistulas (star).
B. Late phase of left vertebral angiography. Pseudoaneurysm (arrow) and arteriovenous fistulas (double arrows) are more visible compared to left vertebral arterial injection.
C. Complete sealing of pseudoaneurysm and arteriovenous fistulas after deployment of stent greft into left vertebral artery.
D. Stenosis of left vertebral artery was noted on follow-up angiogram (arrow). Tip of catheter was in left subclavian artery (arrowhead).
E. After treatment of stenosis by angioplasty, complete recanalisation of lumen was noted on angiogram.
References
1. Inamasu J, Guiot BH. Iatrogenic vertebral artery injury. Acta Neurol Scand. 2005. 112:349–357.
2. Gonzalez A, Mayol A, Gil-Peralta A, Gonzalez-Marcos JR. Endovascular stent-graft treatment of an iatrogenic vertebral arteriovenous fistula. Neuroradiology. 2001. 43:784–786.
3. Felber S, Hankes H, Weber W, Miloslavski E, Brew S, Kuhne D. Treatment of extracranial and intracranial aneurysm and arteriovenous fistula using stent grafts. Neurosurgery. 2004. 55:631–639.
4. Hung CL, Wu YJ, Lin CS, Hou CJ. Sequential endovascular coil embolization for traumatic cervical vertebral AV fistula. Catheter Cardiovasc Interv. 2003. 60:267–269.
5. Waldman DL, Barquist E, Poynton FG, Numaguchi Y. Stent graft of a traumatic vertebral artery injury: case report. J Trauma. 1998. 44:1094–1097.
6. Rich NM, Hobson RW 2nd, Collins GJ Jr. Traumatic arteriovenous fistulas and false aneurysms: a review of 558 lesions. Surgery. 1975. 78:817–828.
7. Halbach VV, Higashida RT, Hieshima GB. Treatment of vertebral arteriovenous fistulas. AJR Am J Roentgenol. 1988. 150:405–412.
8. Mendez CJ, Gonzalez-Llanos G. Endovascular treatment of a vertebral artery pseudoaneurysm following posterior C1-C2 transarticular screw fixation. Cardiovasc Intervent Radiol. 2005. 28:107–109.
9. Chiaradio JC, Guzman L, Padilla L, Chiaradio MP. Intravascular graft stent treatment of a ruptured fusiform dissecting aneurysm of the intracranial vertebral artery: technical case report. Neurosurgery. 2002. 50:213–216.
10. Mourikis D, Chatziioannou A, Doriforou O, Skiadas V, Koutoulidis V, Katsenis K, et al. Endovascular treatment of a vertebral artery pseudoaneurysm in a drug user. Cardiovasc Intervent Radiol. 2006. 29:662–664.
11. Choi JW, Lee JK, Moon KS, Kim YS, Kwak HJ, Joo SP, et al. Endovascular embolization of iatrogenic vertebral artery injury during anterior cervical spine surgery. Spine. 2006. 31:E891–E894.
12. Shintani A, Zervas NT. Consequence of ligation of the vertebral artery. J Neurosurg. 1972. 36:447–450.
13. Saket RR, Razavi MK, Sze DY, Frisoli JK, Kee ST, Dake MD. Stent-graft treatment of extracranial carotid and vertebral arterial lesions. J Vasc Interv Radiol. 2004. 15:1151–1156.
14. Parodi JC, Schonholz C, Ferreira LM, Bergan J. Endovascular stent-graft treatment of traumatic arterial lesions. Ann Vasc Surg. 1999. 13:121–129.
15. Ruckert RI, Rutsch W, Filimonow S, Lehmann R. Successful stent-graft repair of a vertebrojugular arteriovenous fistula. J Endovasc Ther. 2001. 8:495–500.
16. Zingler VC, Strupp M, Brandt T, Herrmann K, Mayer TE. Stent grafting resolved brachial plexus neuropathy due to cervical arteriovenous fistula. Eur Neurol. 2004. 52:250–251.
17. Surber R, Werner GS, Cohnert TU, Wahlers T, Figulla HR. Recurrent vertebral arteriovenous fistula after surgical repair: treatment with a self-expanding stent-graft. J Endovasc Ther. 2003. 10:49–53.
18. Amar AP, Teitelbaum GP, Giannotta SL, Larsen DW. Covered stent-graft repair of the brachiocephalic arteries: technical note. Neurosurgery. 2002. 51:247–252.
19. Redekop G, Marotta T, Weill A. Treatment of traumatic aneurysms and arteriovenous fistulas of the skull base by using endovascular stents. J Neurosurg. 2001. 95:412–419.
20. Priestley R, Bray P, Bray A, Hunter J. Iatrogenic vertebral arteriovenous fistula treated with a hemobahn stent-graft. J Endovasc Ther. 2003. 10:657–663.
21. Sadato A, Satow T, Ishii A, Takayama M, Hashimoto N. Large vertebral arteriovenous fistula treated with stent-grafts: case report. Neurol Med Chir (Tokyo). 2003. 43:250–254.
22. Iakovlev SB, Tissen TP, Bocharov AV, Bukharin EIu. Stent-graft use in endovascular neurosurgery. Zh Vopr Neirokhir Im N N Burdenko. 2006. 2:53–56.
23. Courtheoux P, Guarnieri J, Theron J. Endovascular treatment of a cervical vertebral artery pseudoaneurysm using covered stents. One case report. J Neuroradiol. 2003. 30:109–114.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download