Abstract
We report here on a neonate with congenital cerebellar mixed germ cell tumor, and this initially presented as cerebellar hemorrhage. Postnatal cranial ultrasonography revealed an echogenic cerebellar mass that exhibited the signal characteristics of hemorrhage rather than tumor on MR images. The short-term follow-up images also suggested a resolving cerebellar hemorrhage. One month later, the neonate developed vomiting. A second set of MR images demonstrated an enlarged mass that exhibited changed signal intensity at the same site, which suggested a neoplasm. Histological examination after the surgical resection revealed a mixed germ cell tumor.
Posterior fossa hemorrhage in the full term infant may be associated with breech deliveries, forceps instrumentation and prolonged labor with cranial molding (1). Isolated cerebellar hemorrhage without birth trauma is quite rare (2) and if intracranial hemorrhage occurs in a neonate who is without trauma, then the possibility of coagulation disorders, hypoxia or a hidden tumor should be considered.
Congenital brain tumor may be associated with perinatal hemorrhage with a reported incidence of 14-18% (3, 4). A large amount of hemorrhage may mask the presence of a tumor during the initial evaluation and so the diagnosis can be delayed (5). Approximately 29% to 50% of congenital brain tumors are teratoma, which is the most common brain tumor diagnosed in the first few months of life (4). Congenital mixed germ cell tumor is exceedingly rare (6). We report here on a baby with a congenital cerebellar mixed germ cell tumor and the baby initially presented with cerebellar hemorrhage.
A female infant was transferred to our hospital from a local clinic after the detection of a posterior fossa mass via sonography (US) on the second day of life. She was born after 36+6 weeks of gestation by a normal vaginal delivery. The birth weight was 2,910 gm and the Apgar score at 1 min/5 min after delivery was 8/9. According to the transfer note, prenatal US detected no abnormality in the brain, but this couldn't be reviewed retrospectively because there were no available images. After birth, cephalahematoma at the right parietal area was detected and the initial US was performed to screen for brain injury. Blood testing showed a normal hemoglobulin level and hematocrit. The coagulation studies were also normal: the PT (prothrombin time) was 13.8 seconds (normal range: 11.8-14.3 sec), and the aPTT (activated partial thromboplastin time) was 40.5 seconds (normal range: 32-41.2 sec). US of the brain revealed an echogenic mass at the midcerebellum that was causing mild ventriculomegaly (Figs. 1A, B). The mass showed no vascularity on color Doppler examination. MR imaging was performed to evaluate the nature of the mass on the same day as admission, which demonstrated a 2.0×2.9×2.7 cm sized mass at the cerebellar vermis. The 4th ventricle had collapsed and the proximal ventricles were distended by the mass. The cerebellar mass exhibited a isosignal intensity on the T1-weighted images and dark signal intensity on the T2-weighted images without significant enhancement (Figs. 1C-E). These findings suggested hemorrhage.
Serial US on four, six and 11 days after the first US demonstrated a decreasing echogenicity of the cerebellar mass, and this suggested liquefaction of the hematoma. Because the patient was asymptomatic and there were no clinical problems requiring treatment, she was discharged after 14 days of admission. Brain CT scanning obtained 12 days after discharge demonstrated a slight decrease in the size of the low-attenuated mass, which was not enhanced on the post contrast images (Figs. 1F). One month after the CT scan, the baby developed non-projectile vomiting and brain MR imaging was then carried out. The second set of MR images revealed an enlarged (4.3×4.1×4.3 cm) midline cerebellar mass showing a different signal intensity from the mass on the prior MR images; the mass exhibited T1 and T2 prolongation and contrast enhancement with internal heterogeneity (Figs. 1G, H). Therefore, under the impression of a congenital cerebellar tumor, the baby underwent suboccipital craniectomy with total removal of the mass. Pathological examination revealed a mixed germ cell tumor that predominantly consisted of an immature teratoma and microscopic foci of yolk sac tumor. The histology of the majority of the tumor tissues was immature neuroectodermal tissue; there were a variety of structures derived from all three germ cell layers (Figs. 1I, J). Immunohistochemical study demonstrated the yolk sac tumor, and immature epithelial tissues were reactive to α-fetoprotein (AFP).
Tumor markers such as serum AFP or β-hCG (human chorionic gonadotropin) were not checked before surgery because germ cell tumor was not included in differential diagnosis. After surgical resection, the tumor markers were checked as a baseline study; her serum AFP level was elevated to 366 ng/mL and the β-hCG level was normal. Three weeks later, serum AFP level was normalized.
The patient received three cycles of postoperative chemotherapy. A third set of MR images performed three months after surgical resection showed recurrent tumor at the resection site. Second tumorectomy was performed and the baby then received chemotherapy with a different regimen. The baby is still alive and is undergoing rehabilitation for ataxic cerebral palsy.
Most congenital brain tumors present within the first two months of life and these include teratomas, neuroepithelial tumors, mesenchymal tumors, craniopharyngiomas and hemangioblastomas (3). Although congenital brain tumors represent only 0.5%-1.9% of all pediatric brain tumors, they are responsible for 5-20% of the deaths in this age group (3, 7). Teratoma is the most common congenital brain tumor (4) and it often immature and contains mixed germ cell elements (6). One case with congenital malignant mixed germ cell tumor that involved the frontal lobe and orbit was previously reported in the literature (8). That was the first well-documented case of a congenital intracranial mixed malignant germ cell tumor that was composed of immature teratoma and yolk sac tumor, which was similar to our case.
The diagnosis of a brain tumor in a fetus or neonate is a devastating event, and the correct diagnosis is of critical importance. The imaging findings of a congenital brain tumor include a mass with or without hydrocephalus. A serious concern associated with congenital brain tumor is the occurrence of hemorrhage. The incidence of hemorrhage from a perinatal brain tumor has been reported to be 14-18% (3, 4). There is a consensus in the medical literature that the more primitive the tumor, the more likely it is to bleed (9). If a congenital brain tumor is associated with a large amount of hemorrhage replacing the tumor, then the initial diagnosis may be difficult to make on US as a hemorrhage in the brain may appear an echogenic lesion similar to a congenital tumor. But MR imaging may be helpful to differentiate between a hematoma and a tumor because MR imaging can demonstrate signal characteristics and contrast enhancement. However, in this case, the first MR imaging demonstrated signal characteristics compatible with a hematoma.
It is assumed that a congenital cerebellar tumor was responsible for the hematoma, which might have initially obscured the tumor in our case. It is important to identify the cause of hemorrhage when intracerebral hematoma is identified. Intracranial hemorrhage in a neonate may be associated with trauma, coagulation disorders, hypoxia and a hidden tumor. Trauma is the most common etiology of a hematoma in neonates. Although close follow-up was performed in this case, the initial diagnosis was confused by the history of birth trauma and the imaging findings that suggested a hematoma rather than a neoplasm. Posterior fossa hemorrhages in a neonate with a history of traumatic injury may be accompanied by subdural hematomas or intraparenchymal hemorrhages involving the bilateral cerebellar hemisphere (2). However, an underlying hidden tumor must be considered for patients with isolated midcerebellar hematomas without SDH such as our case, and especially in the absence of any coagulation disorder or clear history of trauma. There are similar prior reports of brain tumors presenting with associated intra-tumor hemorrhage (9, 10). Slow growing tumors may be diagnosed several years after the findings of neonatal intracranial hemorrhage (5).
In summary, we report here on a baby with congenital cerebellar mixed germ cell tumor, and the baby initially presented with cerebellar hemorrhage. If the postnatal imaging suggests there is intracranial hemorrhage in a neonate without trauma, coagulation disorders and hypoxia, then close follow-up is recommended to exclude a hidden tumor.
Figures and Tables
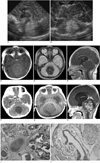
Fig. 1
Mixed germ cell tumor presenting with hemorrhage in newborn.
A, B. Postnatal sagittal sonogram (A) of brain on second day of life demonstrates homogeneous echogenic midline cerebellar mass that is inseparable from echogenic vermis. Coronal Doppler sonogram (B) shows no vascular flow in mass.
C-E. Brain MR images obtained on same day as US demonstrate cerebellar vermian mass exhibiting isosignal intensity on T1-weighted images (C) and this is darkened on T2-weighted image (D). There is no enhancement after administration of contrast medium (E). MR findings were consistent with hematoma.
F. Brain CT obtained 26 days after US demonstrates non-enhancing low-attenuated mass with slight reduction of volume.
G, H. One month after CT scan, mass appears larger and it shows heterogeneous high signal intensity on T2-weighted images (G) and post-contrast T1-weighted images (H). Findings are consistent with neoplasm.
I, J. This tumor consists mainly of immature neuroectodermal tissue, immature cartilage and mesenchymal tissue (I, Hematoxylin & Eosin staining, ×40) as well as yolk sac tumor (arrows) that consists of small neoplastic cells that are separated by myxoid stroma (J, Hematoxylin & Eosin staining, ×200).
References
1. Menezes AH, Smith DE, Bell WE. Posterior fossa hemorrhage in the term neonate. Neurosurgery. 1983. 13:452–456.
2. Von Gontard A, Arnold D, Adis B. Posterior fossa hemorrhage in the newborn -- diagnosis and management. Pediatr Radiol. 1988. 18:347–348.
3. Buetow PC, Smirniotopoulos JG, Done S. Congenital brain tumors: a review of 45 cases. AJR Am J Roentgenol. 1990. 155:587–593.
4. Wakai S, Arai T, Nagai M. Congenital brain tumors. Surg Neurol. 1984. 21:597–609.
5. Nonaka M, Hashira S, Sugamata K, Abe T, Haebara H. [A case of congenital brain tumor diagnosed during follow-up of neonatal intracranial hemorrhage]. No To Hattatsu. 1996. 28:66–71.
6. Raisanen JM, Davis RL. Congenital brain tumors. Pathology (Phila). 1993. 2:103–116.
7. Isaacs H Jr. II. Perinatal brain tumors: a review of 250 cases. Pediatr Neurol. 2002. 27:333–342.
8. Lee JC, Jung SM, Chao AS, Hsueh C. Congenital mixed malignant germ cell tumor involving cerebrum and orbit. J Perinat Med. 2003. 31:261–265.
9. Shimamura N, Asano K, Ogane K, Yagihashi A, Ohkuma H, Suzuki S. A case of definitely congenital glioblastoma manifested by intratumoral hemorrhage. Childs Nerv Syst. 2003. 19:778–781.
10. Rothman SM, Nelson JS, DeVivo DC, Coxe WS. Congenital astrocytoma presenting with intracerebral hematoma. Case report. J Neurosurg. 1979. 51:237–239.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download