Abstract
Meningioma rarely manifests as a subarachnoid hemorrhage (SAH), and invasion directly into a major intracranial artery is extremely rare. To the best of our knowledge, meningioma presenting with an SAH associated with major intracranial arterial invasion has never been reported. We present a case of sphenoid ridge meningotheliomatous meningioma manifesting as an SAH without pathologically atypical or malignant features, due to direct tumor invasion into the middle cerebral artery.
Meningiomas are considered benign tumors that arise from cap cells of the arachnoid granulations (1) and are the most common tumors of the sphenoid ridge (2). It has been well documented that these tumors may invade bone, muscle, dura, or the dural sinuses (3). Despite a tendency to invade venous sinuses, it has been reported that meningiomas do not infiltrate arterial structures (3). Moreover, a subarachnoid hemorrhage (SAH) following major intracranial arterial invasion of meningioma has not yet been reported.
We report a case of a meningotheliomatous type of sphenoid ridge meningioma that manifested as an SAH, caused by tumor invasion into a middle cerebral artery (MCA).
A 53-year-old man presented with a sudden onset of severe diffuse headache followed by dizziness. The patient had no remarkable medical history, except for hypertension, and had not suffered from any recent head trauma. On clinical examination, there was no focal neurological deficit. The systemic blood pressure was normal. Soon after arrival, the patient showed a confused mental status.
A noncontrast CT scan of the head revealed a large amount of SAH in the basal cisterns and left sylvian cistern (Fig. 1A) with a small amount of subdural hemorrhage in the left frontal convexity. On the CT scan, there was also a small hyperdense mass-like lesion seen in the left sphenoid ridge, which showed bony destruction of the left sphenoid ridge with extension into the left anterior middle cranial fossa (Fig. 1B) and the sphenoid paranasal sinus. This lesion showed mild enhancement and was suspected to be in contact with the left MCA as seen on a contrast-enhanced CT scan (Fig. 1C). The possibility of an SAH originating from the ruptured aneurysm was suggested; therefore, cerebral digital subtraction angiography was performed. Cerebral angiography showed no evidence of aneurysms or arteriovenous malformations, but demonstrated a mild focal dilatation at the proximal M2 portion of the left MCA (Figs. 1D, E) and a small tumor blush from the left middle meningeal artery. Since the possibility of an aneurysm was eliminated, an SAH originating from the malignant tumor with vascular invasion was suspected. MR imaging revealed an extraaxial mass lesion in the left sphenoid greater wing with slightly high signal intensity on T2-weighted images and enhancement on contrast-enhanced T1-weighted images (Figs. 1F, G). This lesion showed no definite uptake on a PET scan, suggesting a benign or low-grade tumor (Fig. 1H).
A left frontotemporoparietal craniectomy was performed. There was a soft tissue mass lesion adjacent to the left MCA. The mass was tightly adhered to the left MCA, and resulted in perforation at the MCA bifurcation area (Fig. 1I). The perforated area seemed to be analogous to the focal dilatation at the cerebral angiography. Tumor removal together with primary repair for the perforated MCA was performed.
A pathological examination revealed a white-gray and hemorrhagic myxoid soft tissue mass, which was demonstrated to be a meningotheliomatous meningioma without atypical or malignant features.
Spontaneous intracranial hemorrhage occurs in 3.9% of all brain tumors, mostly in metastatic tumors or malignant gliomas (3-5). The incidence of a spontaneous intracranial hemorrhage associated with a meningioma is 0.5% to 2.4% (3-5). Among the intracranial hemorrhages, a spontaneous SAH is usually considered as a manifestation of an intracranial aneurysm or arteriovenous malformation. An SAH associated with an intraaxial tumor has rarely been reported, comprising an incidence of 1.3% in one report (6). An SAH associated with extraaxial benign tumors such as a meningioma is extremely rare (7). Furthermore, there has not been an earlier report of an SAH manifesting with a meningioma associated with major arterial invasion, as shown in this case.
The exact pathophysiological mechanisms of bleeding in meningiomas are not fully understood. Nevertheless, some suggested hypotheses include rupture from excessive or unusual blood vessels, direct vascular invasion by tumor cells, extensive tumor infarction, stretching and rupture of subdural veins, and the fragility of arterial and venous walls due to rapid tumor growth (3). There are a few reports of a meningioma manifesting as an SAH; however, pathophysiological mechanisms suggested include some of the above described (5, 8), but direct tumor invasion into the major intracranial arteries has never been attributed as a mechanism, such as occurred in this case.
Since meningiomas are known to be incapable of crossing the arachnoid and pial membrane into the brain parenchyma, the major intracranial vessels are usually saved (9) and meningiomas do not infiltrate the arterial structures. There are a few reports describing cavernous sinus meningiomas with carotid artery invasion, and the carotid artery invasion seems to be due to the absence of an arachnoidal plane in the cavernous sinus (5, 9). These investigators also found that, although cavernous meningiomas invade the adventitia of the cavernous carotid artery, they did not appear to invade the media (5, 9).
A meningioma with bony invasion is an uncommon finding. However, it is known that these tumors may invade bony structures, even in the benign meningotheliomatous type (2). In this case, the tumor involved also the sphenoid greater wing with extension into the middle cranial fossa and the sphenoid paranasal sinus.
In this case, the SAH was due to direct invasion of the tumor into the left MCA through the pial coating, which was confirmed by surgery. There are some reports of meningiomas manifesting as an intracranial hemorrhage (3-5, 7, 8). However, to the best of our knowledge, a meningioma presenting with an SAH associated with major arterial invasion has never been reported. This case clearly showed that the major intracranial arterial wall could be invaded and ruptured by a "benign" meningioma that does not have a pathologically atypical or malignant feature.
Figures and Tables
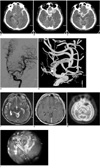 | Fig. 1Initial CT scans and angiographies of 53-year-old man that presented with sudden onset of severe headache.
A. Noncontrast CT axial scan shows large amount of subarachnoid hemorrhage, especially in left sylvian fissure.
B. Contrast-enhanced CT axial scan shows enhancing mass lesion around sphenoid ridge with combined bony destruction.
C. Contrast-enhanced CT axial scan shows that mass is in contact with left middle cerebral artery.
D. Digital subtraction angiography with selective injection of left internal carotid artery reveals no evidence of aneurysms or arteriovenous malformations.
E. Three-dimensional rotational angiography demonstrates mild focal dilatation at proximal M2 portion of left middle cerebral artery.
F. Axial T2-weighted MR image demonstrates hyperintense mass adjacent to left middle cerebral artery.
G. Enhanced axial T1-weighted MR image demonstrates mass with mild enhancement.
H. PET scan shows no definite uptake of mass lesion, suggesting benign or low-grade tumor.
I. Intraoperative photomicrograph. Perforation at just distal portion of left middle cerebral artery bifurcation is noted.
|
References
1. Roser F, Nakamura M, Jacobs C, Vorkapic P, Samii M. Sphenoid wing meningiomas with osseous involvement. Surg Neurol. 2005. 64:37–43.
2. Shaffrey ME, Dolenc VV, Lanzino G, Wolcott WP, Shaffrey CI. Invasion of the internal carotid artery by cavernous sinus meningiomas. Surg Neurol. 1999. 52:167–171.
3. Kim DG, Park CK, Paek SH, Choe GY, Gwak HS, Yoo H, et al. Meningioma manifesting intracerebral hemorrhage: a possible mechanism of haemorrhage. Acta Neurochir (Wien). 2000. 142:165–168.
4. Niiro M, Ishimaru K, Hirano H, Yunoue S, Kuratsu J. Clinico-pathological study of meningiomas with haemorrhagic onset. Acta Neurochir (Wien). 2003. 145:767–772.
5. Bosnjak R, Derham C, Popovic M, Ravnik J. Spontaneous intracranial meningioma bleeding: clinicopathological features and outcome. J Neurosurg. 2005. 103:473–484.
6. Locksley HB, Sahs AL, Sandler R. Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. 3. Subarachnoid hemorrhage unrelated to intracranial aneurysm and A-V malformation A study of associated diseases and prognosis. J Neurosurg. 1966. 24:1034–1056.
7. Pluchino F, Lodrini S, Savoiardo M. Subarachnoid hemorrhage and meningioma. Report of two cases. Acta Neurochir (Wien). 1983. 68:45–53.
8. Yucesoy K, Ozer H, Erdag N, Mertol T. Meningioma presented as subarachnoid haemorrhage: case report. Kobe J Med Sci. 1999. 45:213–219.
9. Kotapka MJ, Kalia KK, Martinez AJ, Sekhar LN. Infiltration of the carotid artery by cavernous sinus meningioma. J Neurosurg. 1994. 81:252–255.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download