Abstract
Congenital coronary sinus anomalies are extremely rare, and they have received relatively little attention. This is probably due to the lack of both clinical symptoms and significant cardiac functional disturbance. We present two cases of a coronary sinus anomaly and briefly review the literature. Recognizing and being familiar with the variations of a congenital coronary sinus anomaly in congenital heart disease may avoid a misinterpretation of cardiac catheterization findings and the troublesome disruption of coronary sinus blood return during the surgical management of cardiac lesions.
Although various types of congenital cardiac malformations have been well studied, anomalies involving the coronary sinus have received relatively little attention; this is probably due to their extreme rarity and that they can occur without clinical symptoms and without a significant cardiac functional disturbance. However, in some situations, the failure to recognize such venous anomalies may cause a misinterpretation of cardiac catheterization findings (1), affecting the distribution of retrograde cardioplegia in patients requiring a cardiopulmonary bypass (2), and may cause a troublesome hemodynamic alteration during cardiac surgery (3).
Most reported cases of coronary sinus anomalies are diagnosed either during a necropsy (1, 4) or by a transesophageal echocardiogram, magnetic resonance imaging (5), and by the use of coronary sinus angiography (5, 6). Multi-detector row computed tomography (MDCT) technology provides a noninvasive alternative to evaluate a cardiac malformation comprehensively. To the best of our knowledge, incidental findings of MDCT of a coronary sinus anomaly have not been previously reported. We present two cases of a congenital coronary sinus anomaly and a brief review of the literature.
A 60-year-old woman visited the cardiac outpatient-department (OPD) of our institute due to persistent chest pain, dizziness and periodic cold sweats that had lasted for years but had recently worsened. The patient denied having any systemic disease in the past. An ECG showed sinus bradycardia and non-specific ST-T changes, and a chest radiograph revealed an unusual enlarged cardiac shadow. To rule out congenital heart disease with an acute coronary syndrome episode, a further evaluation was arranged in the form of a cardiac CT examination.
A cardiac CT was performed using a 64-slice MDCT scanner (Aquilion 64, Toshiba, Japan) with retrospective ECG gating within one single breath-hold. The acquisition protocol was as follows: 0.5 mm section width, 400 msec gantry rotation time, a tube voltage of 120 kVp, and a tube current of 500 mA. Eighty milliliters of non-iodinated contrast medium was injected through an antecubital vein at 4.5 ml/sec and was followed by a 40 ml saline chaser. Using the SureScan and SureStart technologies from Toshiba, the helical scan automatically began when the contrast attenuation in the ascending aorta reached 160 Hounsfield unit (HU). The heart rate averaged 55 beats per minute during the CT scan. Reconstructed images of the three-dimensional volume-rendered images and maximum-intensity-projection images at various phases of the cardiac cycle were performed on a workstation (Vitrea 2, Plymouth, MN).
The MDCT findings revealed a dilated right atrium and right ventricle with abnormal downward displaced insertion of the septal leaflet of the tricuspid valve, suggesting Ebstein's anomaly. A tiny patent foramen ovale (PFO) at the interatrial septum was also suspected. There was no significant luminal stenosis over the three major coronary arteries. Incidental findings of an engorged coronary sinus with atresia of the right atrial ostium and a coexisting abnormal tubular communication to the left atrium were also identified by MDCT (Fig. 1). No other systemic venous anomaly was identified.
A transesophageal echocardiogram was performed and showed a dilated right atrium with lower portion outpouching, downward insertion of the tricuspid valve with moderate tricuspid regurgitation. In addition, a tiny PFO was noted, confirming the MDCT findings as Ebstein's anomaly. A cardiac catheterization was performed and confirmed the insignificant coronary artery disease and adequate left ventricular global performance (ejection fraction = 81%). The patient was placed under medical treatment and scheduled for out-patient follow-up.
A 64-year-old female suffered from vague palpitation and periodic dyspnea for years and sought help at our cardiovascular outpatient department. An ECG showed sinus bradycardia and non-specific ST-T changes. A coronary CTA was arranged for further evaluation.
A coronary CTA was performed using a 64 slice MDCT scanner (Aquilion 64, a unique 64-row Quantum detector from Toshiba, Japan) with retrospective ECG gating. Reconstructed images of the three-dimensional volume-rendered images and maximum-intensity-projection images at various phases of the cardiac cycle were also obtained.
The MDCT findings revealed an abnormal engorged coronary sinus with stenosis of the right atrial ostium and coexisting abnormal tubular communication to the left atrium (Figs. 2A-C). Acceptable vascular lumens of the three major coronary arteries were found. Medical treatment was suggested, and the patient was tracked by outpatient follow-up. No other systemic venous anomaly was identified.
Normal venous development is a process of progression and regression of the three major paired venous systems. The right and left horns of the primitive sinus venosus receive blood from these major veins, namely the vitelline, umbilical, and common cardinal veins, respectively. During the fifth week of development, the left vitelline vein begins to regress. By the tenth week, the left common cardinal vein is occluded, and the left horn has regressed to form the coronary sinus and the oblique vein of 'Marshall,' and the right horn of the sinus venosus becomes incorporated into the right atrium (5). Although an anomaly of the coronary sinus may occur as an isolated condition, it is often associated with other anomalies, either of the various systemic venous anomalies or the anomalous pulmonary venous connection (1).
The coronary sinus normally opens into the right atrium and accounts for 75% of the cardiac venous circulation, returning blood from nearly all regions of the heart, including the septa (2). A classification of coronary sinus anomalies was suggested by Mantini et al. (1), in 1966 including (A) enlargement of the coronary sinus with/without a left-to-right shunt, (B) absent coronary sinus, (C) atresia of the right atrial coronary sinus ostium, and (D) hypoplasia of the coronary sinus (Figs. 2D-K).
Enlargement of the coronary sinus (Figs. 2D-G) should raise a suspicion of the possibility of anomalous systemic venous return into the coronary sinus, or the possibility of an anomalous left-to-right shunt either from high-pressure shunting, such as a coronary artery-coronary sinus fistula, or low-pressure shunting from the pulmonary artery and left atrium. An unusually large communication between the left atrium and coronary sinus may occur, and may be misinterpreted as an atrial septal defect in the catheterization findings (1) that can cause confusion for corrective cardiac surgery.
The rare type of absence of the coronary sinus (Fig. 2H) is always associated with a persistent left superior vena cava (PLSVC) connection to the left atrium, an atrial septal defect and possibly other additional anomalies. It usually has a right-to-left shunt at the left atrial level as part of the complex functional abnormality. In another extremely rare type of hypoplasic coronary sinus (Fig. 2I), some of the cardiac veins empty individually into the atrial chambers through dilated thebesian channels due to a failure in joining the coronary sinus. There is usually no major functional significance for such coronary sinus hypoplasia cases (1).
Atresia of the right atrial ostium of the coronary sinus may occur as an isolated anomaly or in association with other cardiac malformations (Figs. 2J, K). If a functional PLSVC exists, blood returns in a retrograde direction, passing upward to the left superior vena cava, the left innominate vein, the right superior vena cava, and eventually into the right atrium. Therefore, special care should be taken in cases with a PLSVC and a dilated coronary sinus as dividing or ligating the PLSVC during surgical management of cardiac lesions may disrupt the coronary sinus venous return, leading to myocardial edema, ischemia, and necrosis with a poor patient outcome (3).
In cases of atresia or stenosis of the right atrial ostium without coexistence of the left superior vena cava, the blood from the coronary sinus into the related atria may pass through alternative pathways such as a window to the left atrium, multiple enlarged thebesian veins, or through the levoatriocardinal veins that connect the coronary sinus and the left atrium (1, 2) (Figs. 2J, K). The term 'levoatriocardinal vein' was used by Edwards and DuShane to indicate an anomalous connection between the left atrium or a pulmonary vein to any derivative of the cardinal venous system (7). It was thought that this levoatriocardinal vessel persisted in response to a partially or totally obstructed ostium of the coronary sinus during early development, serving as a collateral outflow channel for the coronary sinus.
It is important to be aware that in cases of right atrial ostial atresia/stenosis of the coronary sinus with coexistence of PLSVC or the levoatriocardinal vein, the anomalous venous channels usually serve as the only way or the main collateral outflow for the coronary sinus. Therefore, an alteration of these anomalous channels may lead to significant coronary venous obstruction (1, 3).
In a board range of cardiac surgeries, retrograde coronary sinus cardioplegia perfusion (RCP) is widely used as a method of myocardial protection. RCP is found to be especially beneficial in cases with severe coronary artery disease that can alter the distribution of antegrade cardioplegia, and is effective in cases involving aortic regurgitation or an open aortic root (2). However, it is difficult to perform RCP in patients with a right atrial ostial atresia/stenosis of the coronary sinus preoperatively, and in other types of coronary sinus anomalies, the efficiency and distribution of a RCP may be affected, which leads to partial or poor myocardial protection.
In the presented two cases, the MDCT findings revealed an abnormally enlarged coronary sinus; the right atrial ostium was either stenostic or atresia. Communication of an abnormal tubular structure, probably the levoatriocardinal vein, was found between the coronary sinus and the left atrium. However, no functional PLSVC existed in both cases. According to the different contrast density within the left atrium, right atrium and the coronary sinus, a left-to-right flow through the levoatriocardinal vein was found in case 2 with right atrial ostial stenosis, and the increased flow resulted in an abnormally dilated and engorged coronary sinus. A bi-directional flow (both left-to-right and right-to-left shunt) was suspected in the patient of case 1 with right atrial ostial atresia, due to the small amount of high-density contrast within the coronary sinus and in the small levoatriocardinal channel. We speculated that there might be other coronary sinus outflow pathways in this case of ostial atresia, as the size of the levoatriocardinal vein with bi-directional flow was not as large as we expected. The other blood return may pass through the thebesian veins that were hidden within the myocardium and were grossly invisible. The association between the Ebstein's anomaly and coronary sinus ostial atresia in the case 1 patient is still to be determined due to the broad pathological-anatomical and clinical spectrum of Ebstein's anomaly that have been identified in the reported literature and the differences in presentation for different patients.
Although there is no significant functional disturbance in either of the two cases, left-to-right shunting into coronary sinus-related increased inflow combined with the right atrial ostial atresia/stenosis related obstructive outflow may raise the possibility of myocardium congestion. The clinical significance between the coronary sinus anomaly and the persistent chest discomfort of the two patients remains to be determined.
In summary, the use of MDCT provides a noninvasive alternative for pre-operative evaluation of congenital heart disease. A coronary sinus anomaly may occur as an isolated anomaly or more commonly as a component of congenital heart disease. Recognizing and being familiar with the variations of congenital coronary sinus anomalies may avoid misinterpretation of cardiac catheterization findings in the preoperative evaluation of cardiac lesions, and may help in the pre-operative planning of retrograde cardioplegia, and may avoid troublesome hemodynamic disruption of coronary sinus blood return during cardiac surgery.
Figures and Tables
Fig. 1
Congenital coronary sinus anomaly in 60-year-old woman with Ebstein's anomaly.
A, B. Dorsal view of reconstructed volume-rendered image (A) and maximum-intensity-projection image (B) reveal normal coronary sinus (arrow) drain into right atrium (RA). RA = right atrium; LA = left atrium
C. Dorsal view of reconstructed volume-rendered image reveals abnormal engorged coronary sinus (long white arrow) without gross communication with right atrium (RA). There was small tortuous vascular channel (short black arrow) drain into left atrium (LA). Atrialization portion of right ventricle also could be identified (black star).
D. Series maximum-intensity-projection images confirmed that there was no communication between abnormally engorged coronary sinus (long white arrows) and right atrium (RA). In addition, there was small tortuous vascular channel with high contrast density (black arrows) located between engorged coronary sinus and right atrium (RA). Small foci of high contrast density within coronary sinus also had been found (black arrows).
E. Post-processing oblique-dorsal view of reconstructed volume-rendered image with total removal of right atrium revealed that this small tortuous vascular channel (black arrows) connected coronary sinus (CS) and left atrium (LA).
RA = right atrium; LA = left atrium; CS = coronary sinus
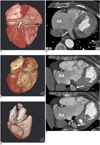
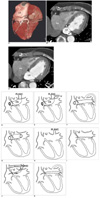
Fig. 2
Coronary sinus anomaly with stenosis of right atrial ostium and coexisting levoatriocardinal vein communication to left atrium in 64-year-old woman.
A. Dorsal view of reconstructed volume-rendered image reveals abnormal engorged coronary sinus (long white arrow) without grossly visible communication with right atrium (RA). There was engorged vascular channel (black star) arising from coronary sinus that was highly suggestive of communication with left atrium (LA).
B. Maximum-intensity-projection image revealed that aforementioned vascular channel (black star) was connected to left atrium (LA) with large opening. Evidence of large left-to-right shunting is also noted according to equal high-contrast density within CS and LA. Stenostic end of coronary sinus into right atrium (RA) was also seen (black arrow).
C. Sequential maximum-intensity-projection image next to B demonstrates stenostic right atrial ostium (black arrow) of coronary sinus. RA = right atrium, LA = left atrium
D-G. Illustration of enlargement of coronary sinus (CS) associated with (D) a persistent left superior vena cava (PLSVC); (E) PLSVC and other anomalous systemic venous return; (F) anomalous left-to-right shunt from left atrium; (G) unusually large communication between left atrium and coronary sinus (modified from Mantini and colleagues (1)).
H. Illustration of absence of coronary sinus, which is always associated with persistent left superior vena cava (PLSVC) and atrial septal defect (modified from Mantini and colleagues (1)).
I. Illustration of hypoplasic coronary sinus; cardiac veins failed to join coronary sinus and emptied into atrial chamber through dilated thebesian channels (modified from Mantini and colleagues (1)).
J. With functional persistent left superior vena cava (PLSVC), blood returns in retrograde direction, passing upward to persistent left superior vena cava (PLSVC), left innominate vein, right superior vena cava, and eventually into right atrium.
K. Without persistent left superior vena cava (PLSVC), blood returns through levoatriocardinal vein then into left atrium (modified from Mantini and colleagues (1)).
References
1. Mantini E, Grondin CM, Lillehei CW, Edwards JE. Congenital anomalies involving the coronary sinus. Circulation. 1966. 33:317–327.
2. Ruengsakulrach P, Buxton BF. Anatomic and hemodynamic considerations influencing the efficiency of retrograde cardioplegia. Ann Thorac Surg. 2001. 71:1389–1395.
3. Jha NK, Gogna A, Tan TH, Wong KY, Shankar S. Atresia of coronary sinus ostium with retrograde drainage via persistent left superior vena cava. Ann Thorac Surg. 2003. 76:2091–2092.
4. Rose AG, Beckman CB, Edwards JE. Communication between coronary sinus and left atrium. Br Heart J. 1974. 36:182–185.
5. Miraldi F, di Gioia CR, Proietti P, De Santis M, d'Amati G, Gallo P. Cardinal vein isomerism: an embryological hypothesis to explain a persistent left superior vena cava draining into the roof of the left atrium in the absence of coronary sinus and atrial septal defect. Cardiovasc Pathol. 2002. 11:149–152.
6. Neuser H, Kerber S, Schumacher B. Images in cardiovascular medicine. Fistulous communication between coronary sinus and left atrium. Circulation. 2002. 106:E137–E138.
7. Edwards JE, DuShane JW. Thoracic venous anomalies. Arch Pathol. 1950. 49:517–537.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download