Abstract
Although intracranial dural metastasis of Ewing's sarcoma is a very rare finding, its imaging characteristics are similar to those of its primary form in the central nervous system. Thus, this tumor must be considered in the differential diagnosis of extra-axial dural masses.
Ewing's sarcoma is a malignant bone tumor that can occur anywhere in the body, but it is most commonly observed in the long bones of the arms and legs, the pelvis and in the chest. The predominant sites of metastasis include the lung (38%), bone (including the spine; 31%), and the bone marrow (11%) (1). Metastasis of Ewing's sarcoma to the central nervous system (CNS) is relatively rare, and most of the previous reports have demonstrated involvement of the bony calvarium or brain parenchyma (2). We describe here the imaging findings of dural metastasis of Ewing's sarcoma, and these imaging findings have not been previously reported on in the medical literature.
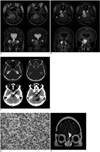
A 21-year-old woman presented in 1996 with right arm pain and weakness. She underwent total laminectomy at C7-T1 with gross total removal of the intradural tumor and partial removal of the extradural tumor. The pathologic diagnosis was Ewing's sarcoma, and it showed the expression of MIC-2 antigen according to immunohistochemistry. The patient presented with recurrent right side weakness the following year and so she underwent resection of the recurrent mass followed by chemotherapy and radiation treatment. No metastatic lesion was found at the time of the first or second operation. The patient was healthy until August 2004 when she complained of headache. No recurrent tumor was found in the spine at that time. In March 2005, she presented with slowly progressing left side weakness and paresthesia. MRI was performed at 3.0 T, and this showed a mass in the right cerebellopontine angle cistern with extension into the ipsilateral Meckel's cave (Figs. 1A, B). The mass showed a slightly heterogeneous lobulated contour with similar or slightly higher signal intensity than the cortex on the T2-weighted images, with heterogeneous enhancement. Several spots of dark signal-intensity were noted within the mass on MRI, suggesting the presence of hypervascularity, hemorrhage or calcifications. Edema was noted in the pons and cerebellum adjacent to the mass. Noncontrast CT showed a spot of subtle high attenuation within the mass, suggesting the possibility of calcification or hemorrhage (Fig. 1C). CT also demonstrated a high-attenuation curvilinear area near the cerebellum, and this showed low signal intensity on T2-weighted imaging and no enhancement (Fig. 1A, B). The ipsilateral internal auditory canal and petrous bone were free of tumor on both CT and MRI (Fig. 1A-C). Catheter angiography showed no blood supply from the meningeal arteries. Surgical resection was performed via a suboccipital approach with partial removal of the ipsilateral mastoid. No petrous bone or adjacent brain parenchyma involvement was found. The surgeon observed focal hemorrhage within the mass. Complete resection of the mass was done and this was followed by radiation treatment. The pathologic diagnosis was a metastatic Ewing's sarcoma with a MIC-2 antigen expression (Fig. 1D). The curvilinear area of high attenuation on CT was determined to be fibrosis on the pathologic examination. The patient again presented with headache in January 2006. MRI revealed a new enhancing lesion abutting the anterior falx cerebri, and left fovea ethmoidalis was also noted (Fig. 1E). The FDG PET scan demonstrated increased metabolism in the mass, suggesting the recurrence of tumor. Radiation treatment was performed for the lesion.
Ewing's sarcoma has a propensity to metastasize to the lung, bone and bone marrow. This tumor can also involve the CNS with a relatively low incidence (5 of 80 patients, 6.3%) being observed in a previous study (3). There are two reported principal modes of metastatic spread of Ewing's sarcoma to the CNS. The first is direct extension from the skull, which may be the site of both primary and secondary Ewing's sarcoma (4). Shuper et al. have reported that metastases to the brain directly extended from the skull in two of seven patients with brain metastasis (3). Alternatively, Ewing's sarcoma may reach the CNS via hematogenous spread. In their case series, Shuper et al. also reported that five of seven patients with metastasis of Ewing's sarcoma to the brain had parenchymal lesions that were separate from the skull and meninges, which suggested hematogenous spread of tumor. Other studies investigating CNS involvement of Ewing's sarcoma have reported that spread through direct extension from the skull is more frequent than hematogenous spread (5, 6).
Metastasis to the dura accounts for 9% of all CNS metastases (7). Various malignant neoplasms, including breast cancer, prostate cancer, melanoma, multiple myeloma, malignant lymphoma and leukemia, can secondarily involve the dura. However, dural metastasis of Ewing's sarcoma has rarely been reported on. Only one case of dural metastasis of Ewing's sarcoma has been described in a report of an autopsy series (8). In that report, the lesion also involved the skull with diffuse, massive epidural and subdural plaque-like nodules. Therefore, the dural nodules might have extended from the skull, which is a frequent metastatic site for this tumor. A lack of imaging findings in that case makes it difficult to determine the origin of the dural lesion. In our patient, no abnormality in the skull abutting the metastatic tumor was noted on imaging or during surgery.
Primary Ewing's sarcoma of the central nervous system has rarely been reported on. The imaging characteristics of this rare tumor in an extra-axial location consist of high attenuation on CT and low signal intensity on T2-weighted MRI (4). Hemorrhagic components, dural extension and contrast enhancement were also reported. All three reported cases showed bone involvement, indicating that the origin of this rare tumor was the skull and not the dura (4). Another radiology report also showed similar imaging findings, but without skull involvement (9), which is very similar to the findings of our patient. It is not possible to say whether the resected tumor was a primary Ewing's sarcoma or a secondary tumor because we did not confirm chromosomal translocation by performing fluorescent in situ hybridization. However, the subsequent recurrence might suggest that our patient had a metastatic tumor because primary Ewing's sarcoma has been reported to show a more favorable outcome (10).
Our initial differential diagnosis included more common tumors such as a meningioma. A schwannoma was also thought to be a possibility following catheter angiography due to the lack of blood supply from the meningeal arteries. Relatively lower signal intensity within the mass on T2-weighted imaging usually suggests hypercellularity, as is generally found in meningioma, lymphoma or leukemia. Ewing's sarcoma is also in the category of hypercellular tumors, and this hypercellularity is evident in both primary and secondary tumors. The lack of bone involvement supports the possibility of hematogenous spread of the dural lesion in our patient. In addition, although it was not confirmed pathologically, a new dural lesion was detected on follow-up imaging, raising the possibility that the prior lesion had the same mode of spread.
In conclusion, dural metastasis of Ewing's sarcoma is very rare and its imaging characteristics are similar to those of a primary tumor, which mimic the findings of a schwannoma or meningioma. Despite its rarity, secondary Ewing's sarcoma may be included in the differential diagnosis of extra-axial dural masses.
Figures and Tables
Fig. 1
The T2-weighted (A) and postcontrast T1-weighted images (B) show a mass in the right cerebellopontine angle cistern with extension into the ipsilateral Meckel's cave. The mass shows a slightly heterogeneous lobulated contour with similar or slightly higher signal intensity than the cortex on the T2-weighted images, with heterogeneous enhancement. Edema is noted in the pons and cerebellum adjacent to the mass. Noncontrast CT shows spots of subtle high-attenuation within the mass (arrows), suggesting the possibility of calcification or hemorrhage. Also seen is a high-attenuation curvilinear area between the mass and the cerebellum (curved arrow), which was determined to be fibrosis on the pathologic examination (C). The ipsilateral internal auditory canal and petrous bone are free of tumor on both the CT and MRI (A-C). Immunohistochemistry demonstrates the MIC-2 antigen expression (D). Follow-up MRI reveals a new enhancing lesion abutting the anterior falx cerebri and left fovea ethmoidalis, and this suggests metastasis (E).
References
1. Pizo P, Poplack D. Ewing's sarcoma of bone and soft tissue: principles and practice of pediatric oncology. 1996. 3rd ed. Philadelphia: JB Lippincott;840–841.
2. Postovsky S, Ash S, Ramu IN, Yaniv Y, Zaizov R, Futerman B, et al. Central nervous system involvement in children with sarcoma. Oncology. 2003. 65:118–124.
3. Shuper A, Cohen IJ, Mor C, Ash S, Kornreich L, Zaizov R. Metastatic brain involvement in Ewing family of tumors in children. Neurology. 1998. 51:1336–1338.
4. Li WY, Brock P, Saunders DE. Imaging characteristics of primary cranial Ewing sarcoma. Pediatr Radiol. 2005. 35:612–618.
5. Mehta Y, Hendrickson FR. CNS involvement in Ewing's sarcoma. Cancer. 1974. 33:859–862.
6. Marciani MG, Stefani N, Peroni L, Stefanini F, Tarantino U, Gigli GL, et al. Intracerebral metastasis in Ewing's sarcoma. Acta Neurol Belg. 1990. 90:149–156.
7. Meyer PC, Reah TG. Secondary neoplasms of the central nervous system and meninges. Br J Cancer. 1953. 7:438–448.
8. Kleinschmidt-DeMasters BK. Dural metastases. A retrospective surgical and autopsy series. Arch Pathol Lab Med. 2001. 125:880–887.
9. Pekala JS, Gururangan S, Provenzale JM, Mukundan S Jr. Central nervous system extraosseous Ewing sarcoma: radiologic manifestations of this newly defined pathologic entity. AJNR Am J Neuroradiol. 2006. 27:580–583.
10. Dirks PB, Harris L, Hoffman HJ, Humphreys RP, Drake JM, Rutka JT. Supratentorial primitive neuroectodermal tumors in children. J Neurooncol. 1996. 29:75–84.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download