This article has been corrected. See "Erratum" in Volume 9 on page 190.
Abstract
Objective
This prospective study evaluated the ability of indirect 16-row multidetector CT venography, in comparison with Doppler sonography, to detect deep vein thrombosis after total hip or knee replacement.
Materials and Methods
Sixty-two patients had undergone orthopedic replacement surgery on a total of 30 hip joints and 54 knee joints. The CT venography (scan delay time: 180 seconds; slice thickness/increment: 2/1.5 mm) and Doppler sonography were performed 8 to 40 days after surgery. We measured the z-axis length of the beam hardening artifact that degraded the image quality so that the presence of deep vein thrombosis couldn't be evaluated on the axial CT images. The incidence and location of deep vein thrombosis was analyzed. The diagnostic performance of the CT venograms was evaluated and compared with that of Doppler sonography as a standard of reference.
Results
The z-axis length (mean±standard deviation) of the beam hardening artifact was 4.5 ± 0.8 cm in the arthroplastic knees and 3.9 ± 2.9 cm in the arthroplastic hips. Deep vein thrombosis (DVT) was found in the popliteal or calf veins on Doppler sonography in 30 (48%) of the 62 patients. The CT venography has a sensitivity, specificity, positive predictive value, negative predictive value and accuracy of 90%, 97%, 96%, 91% and 94%, respectively.
Major orthopedic surgeries such as total hip arthroplasty (THA) and total knee arthroplasty (TKA) are commonly performed surgical procedures. However, these procedures have an increased risk of venous thromboembolism with an incidence of up to 84% (1-8). Deep vein thrombosis (DVT) may progress to a potentially fatal pulmonary embolism and also secondary complications such as postthrombotic syndrome, recurrent DVT and chronic pulmonary hypertension (9, 10). Therefore, the early detection and proper management of DVT is important for preventing unexpected complications.
The diagnostic modalities for detecting the DVT that develops after total joint replacement includes conventional venography (11, 12), Doppler sonography (1-3,13-26), MR venography (27) and radionuclide venography (28). Compared with conventional venography, Doppler sonography has the advantage of being portable, painless and noninvasive, there are no side effects and it is a less expensive screening and surveillance tool for managing DVT (13). It was also reported to be a good examination method with an overall sensitivity and specificity of 86% and 97%, respectively, compared with conventional venography (13).
To the best of our knowledge, CT had not been used to detect DVT after total hip or knee arthroplasty mainly because of the beam hardening artifacts that are caused by the arthroplastic joint materials. Therefore, the aim of this prospective study was to compare the ability of indirect 16-row multidetector row CT venography with that of Doppler sonography to detect DVT after TKA or THA.
The institutional review board approved the study protocol. Between June 2004 and February 2005, among all the patients who underwent total hip or knee arthroplasty at our hospital, the 62 patients (19 men and 43 women; age range, 28-83 years; mean age, 63 years) that had both CT venography and Doppler sonography performed after surgery were enrolled in this study. THA was performed in 29 patients unilaterally (n = 28) or bilaterally (n = 1). TKA was performed in 34 patients unilaterally (n = 14) or bilaterally (n = 20). In only one patient, both THA and TKA were performed unilaterally during a single operation. Overall, 30 hips and 54 knees were examined after surgery with using noninvasive color duplex Doppler ultrasound flow scanning and indirect 16-row multidetector row CT venography.
CT venography was performed 8-29 days (mean: 12 days) after surgery to detect DVT. According to Eilas et al. (1), most DVT occurs at the 8th to 13th day after major joint surgery. Doppler sonography was carried out within two weeks (mean: 2 days) after CT venography. For only eight of 62 patients, the interval between the two examinations was more than four days (range: 4 to 11 days). Of the two radiologists who were experienced in vascular imaging, one interpreted the result of CT venography and the other separately performed the Doppler sonography. The radiologists who performed the CT venography were unaware of the results of the Doppler sonography and vice versa.
The status of the arteries and veins in the bilateral lower extremity was evaluated preoperatively via Doppler sonography in all the patients within 35 days (mean: 9). No significant arterial stenosis or DVT was detected in any patient. At our hospital, no pharmaceutical prophylaxis was administered for DVT.
The MDCT scans were performed on a Somatom Sensation 16 plus helical CT scanner (Siemens Medical Systems, Erlangen, Germany). After obtaining the topogram from the diaphragm to the feet, CT venography was performed with a detector collimation of 16 × 1.5 mm, a table feed of 24 mm, 120 kV, 200 effective mAs, a rotation time of 0.37 seconds and with 160 mL of contrast media (Omnipaque; Amersham Health, Cork, Ireland) at a flow of 4 mL/s, and the contrast media was administered by an automated injector (MCT Plus; Medrad, Pittsburgh, PA) through the antecubital vein. Immediately after the contrast injection, 40 mL of normal saline was pushed with a flow rate of 2 mL/s. The start delay time was determined with using bolus tracking software. The region of interest was placed on the abdominal aorta at the level of the second lumbar vertebra. Fifteen seconds after the arrival of 100 Hounsfield units (HU), the CT scan was begun in the craniocaudal direction. One hundred-eighty seconds after the start of the contrast material, indirect CT venography was done from the diaphragm down to the feet. A delay of 180 seconds was chosen according to the time-density curves of the lower limb veins as reported by Szapiro et al. (29), who showed that the optimal window for CT venography was obtained between 210 and 240 seconds for the calf level and between 180 and 300 seconds for the above-knee veins. No further contrast material was administered for the venous phase. Care Dose 4D (Siemens Medical Systems, Erlangen, Germany) was also used to further decrease the radiation doses to the patient.
An informed consent was obtained from either the patients or their relatives. The CT venography was reconstructed with a slice width of 2 mm and an increment of 1.5 mm. The reconstructions were performed with a soft-tissue reconstruction kernel (B30F medium smooth). The window was adjusted depending on the vessel opacification. The criterion for deep vein thrombosis included non-enhanced, low-attenuated lesions within the deep veins of the lower extremities.
In all the patients, bilateral leg sonography was performed with a 5 to 10 MHz linear array transducer (VST MASTER, Diasonics, Milpitas, CA; or ATL5000, Philips Medical Systems, Bothell, WA) by an experienced radiologist. The common and superficial femoral veins were assessed with the patient in the supine position. The popliteal vein was examined with the patient turned to either on their side or with the patient in the prone position. A color Doppler ultrasound examination of the calf veins was then performed with the patient in the prone position and with a pillow under the feet. Transverse and longitudinal gray scale images with and without compression as well as the transverse and longitudinal color and spectral Doppler sonography, including the flow accentuation maneuvers, were performed. The main diagnostic criterion used for DVT was the loss of venous compressibility. The findings were compared with the CT venographic results after the examination. If there were any divergent results, then further sonographic analysis was carried out immediately by repeating the examination. The typical sonographic examination time was from 10 to 20 minutes for each leg.
The quality of the CT venographic images was initially evaluated both subjectively and objectively. The image quality of the CT venograms was graded as good, sufficient or insufficient based on visual analysis. 'Good' means that the degree of venous enhancement was similar to that of the adjacent arterial enhancement. 'Insufficient' means that the degree of venous enhancement was similar to that of the adjacent muscular enhancement. 'Sufficient' means that the degree of venous enhancement was between those of the adjacent arterial and muscular enhancements. For objective analysis of the image quality, the attenuation of the superficial femoral vessels (artery and vein) and the adjacent muscle at the mid-femur level was measured. For this, we placed a small round region of interest within the selected subject. The differences in the attenuation values of the superficial femoral vein and adjacent muscle were analyzed statistically with using Student's t-test.
We then measured the z-axis length of the beam hardening artifact that degraded the image quality so that the presence of DVT couldn't be evaluated on the axial CT images. We evaluated the mean length and range of the beam hardening artifact and we compared the results between the arthroplastic hips and knees.
The incidence and location of the DVT was then analyzed depending on the arthroplasty method (THA vs. TKA) and the operated limb (unilateral vs. bilateral). The incidence was counted as the number of the patients who had DVT. The location was divided into 1) the inferior vena cava, 2) the common and external iliac veins, 3) the superficial femoral vein, 4) the popliteal vein and 5) the calf vein. However, each thrombus was not matched between the Doppler sonography and the CT venography on a lesion-to-lesion basis. Only the presence of DVT was recorded within each selected vein.
Finally, the diagnostic performance of CT venography compared with that of Doppler sonography was examined by determining the sensitivity, the specificity, the positive and negative predictive values and the accuracy.
In the 62 patients, the image quality of the CT venograms was good (n = 59) or sufficient (n = 3), as judged subjectively. Objectively, at the mid-femur level, the attenuations (mean ± standard deviation [SD]) of the superficial femoral vein and artery were 140 ± 23 HU and 161 ± 26 HU, respectively. On the other hand, the attenuation of the adjacent muscle was 65 ± 8 HU (mean ± SD). Therefore, the difference between the attenuation values of the superficial femoral vein and adjacent muscle was significant (p< 0.001).
The z-axis length (mean ± SD) of the beam hardening artifact was 4.5 ± 0.8 cm (range: 3.0-6.0 cm) in the arthroplastic knees and 3.9 ± 2.9 cm (range: 0-10.0 cm) in the arthroplastic hips. Therefore, the extent of beam hardening artifact in the arthroplastic hips was more variable than that in the arthroplastic knees.
Deep vein thrombosis was found in the calf veins by Doppler sonography in 30 (48%) of the 62 patients who were enrolled in this study (Table 1). In two patients, the calf vein DVT extended into the popliteal vein. There was no DVT in the iliofemoral veins in our study. DVT was detected by Doppler sonography in 42 operated extremities and in one non-operated extremity (Figs. 1, 2). All the DVTs were non-occlusive at the time of the diagnosis and the patients were asymptomatic. In our study, the incidence of DVT was higher after TKA (76%, 25 of 33 patients) than after THA (14%, 4 of 28 patients); this was not analyzed statistically.
Discrepancies between the CT venograms and Doppler sonography were present in four patients. In three patients, CT venography did not initially detect the DVT despite the presence of DVT as detected by Doppler sonography. However, in these three patients, the DVT was retrospectively found on the CT venography (Fig. 3). In one patient, the CT venography showed a suspicious focal DVT in the calf veins (Fig. 4), but there was also no DVT noted in the calf veins on the repeated sonographic examinations. Based on the results of Doppler sonography, the CT venography has a sensitivity, specificity, positive predictive value, negative predictive value and accuracy of 90%, 97%, 96%, 91% and 94%, respectively, for the diagnosis of DVT after major orthopedic arthroplasty (Table 2).
Since the advent of MDCT, the diagnostic ability of CT venography to detect DVT in the lower extremities was reported to be comparable to Doppler sonography (30-32). These findings were mainly based on the significant difference in attenuation between the deep vein and the perivenous muscle. In this study, the deep vein was easily differentiated from the perivenous muscular tissue after contrast enhancement by visual analysis, as well as by measurement of the attenuation.
CT has not been used as a diagnostic imaging modality for detecting DVT after major joint arthroplasty because of the general disadvantages such as the radiation hazard and contrast-induced nephropathy. In addition, the beam hardening artifacts that develop due to the artificial joint materials strongly hampers the use of CT. Streak or beam hardening artifacts result in hypodense or hyperdense streaks in the neighboring structures. However, such artifacts can be distinguished from DVT because they extend through the vessel into the perivascular tissue and they show direct contrast to a clot, which is rounded and can be seen on the consecutive images (33). In the TKA patients, beam-hardening artifacts consistently occurred in a limited area (less than 6.0 cm in our study) in which the artificial joint was present on the axial image. For the THA patients, the artifact involved the very long segment from the femoral head to the middle femur shaft; however, the artifact did not degrade the image quality so much. The reasons for this could be inferred that the meaningful length of the artifact in the arthroplastic hips was rather less than that in the arthroplastic knees. First, the area of the artificial joint material on the axial CT images was smaller in the arthroplastic hips. Second, the distance between the adjacent major vein and the artificial joint material was longer in the arthroplastic hips. Therefore, the beam hardening artifact did not disturb the image quality along the very long segments in the arthroplastic hips and knees.
For most of the patients of this study, DVT occurred exclusively at the calf veins. The importance of an isolated calf vein DVT as the cause of a clinically important pulmonary embolism or persistent lower extremity symptoms has been a subject of considerable debate in the medical literature (30). According to Wang et al., a calf vein DVT after TKA disappears spontaneously with time (12). Among the 48 patients in Wang's study, no recurrent DVT and no proximal propagation or embolism developed. However, Delis et al. (34) reported that a calf vein thrombosis might propagate to the proximal veins; 50% of the calf clots totally lysed within four months, yet reflux developed in at least 75% of the limbs with DVT.
Most DVT initially occurs at the calf vein, and it then propagates to the femoropopliteal veins. An isolated DVT within the femoral or iliac vein is known to be a rare condition. Therefore, beam-hardening artifacts were not problematic when making the diagnosis of DVT, although some portion around the hip or knee joint could not be evaluated due to the degradation of the image data. Before the beginning of this study in our hospital, the sonographic examination included an evaluation of the deep veins only around the hip and knee joints. At that time, the incidence of DVT was quite low, and particularly in the asymptomatic patients.
The incidence of DVT was significantly greater after TKA than after THA (Table 1). These results are comparable to those reported by Leutz and Stauffer et al. (14). The main causes might be immobilization, soft tissue swelling and inflammation after joint surgery.
It is unclear if performing bilateral sonography offers any real advantage over sonography of the operated leg alone. However, the risk of an isolated DVT in the non-operated leg is approximately 4% to 5% (11). In the clinical trials that aimed at evaluating the efficacy of thromboprophylaxis for major orthopedic surgery, bilateral venography was noted to reduce the risk of undiagnosed DVT in the non-operated leg (11).
In our experience, sonographic evaluation of the calf veins was time-consuming and dependent to the operator's experience. In addition, the small isolated thrombi confined within the calf vein were easily ignored. For those reasons, it was reported that Duplex ultrasound has a low sensitivity (15, 18, 19). However, Doppler sonography has been used as the primary imaging modality for detecting DVT after joint arthroplasty because of its wide availability and the patient's safety. In our study, the diagnostic performance of CT venography was comparable to that of Doppler sonography, but several limitations were present. First, we didn't perform a gold standard modality such as conventional ascending venography. Further comparative studies between CT venography and conventional ascending venography may well be needed in the future. Second, a time interval of more than four days was present between the Doppler sonography and CT venography for several patients. Because the examination findings of the patients did not show discrepancies between the two imaging modalities, it seemed that the results of our study were not influenced by the long time intervals between the two different examinations.
Figures and Tables
Fig. 1
A 63-year-old female underwent left total knee arthroplasty.
A. Note the degradation of the image quality of the 3.5 cm-long segment (between the two arrows) in the popliteal fossa on the three-dimensional volume rendered image.
B. An axial CT image shows the non-enhancing, low-attenuated lesions surrounded by contrast material within the left calf veins (asterisks).
C. Color Doppler sonography reveals a hypoechoic lesion partially obstructing the left calf vein. There were blood flow signals surrounding the lesion.
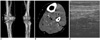
Fig. 2
A 46-year-old female underwent left total hip arthroplasty.
A. The three-dimensional volume rendering image shows the degradation of image quality along the long segment (between the two arrows) due to beam hardening artifact by the arthroplastic joint material. However, the entire common and superficial femoral veins, except for a 2 cm-long segment, could be evaluated for whether deep vein thrombosis was present or not.
B. An axial CT image shows beam hardening artifact traversing the adjacent superficial femoral vein (arrow).
C. A coronal image shows non-enhancing, low-attenuated lesions surrounded by contrast material within the left calf veins (arrows).
Doppler sonography confirmed the deep vein thrombosis (not shown).
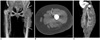
Fig. 3
A 54-year-old female underwent left total hip and knee arthroplasty simultaneously.
A, B. Doppler sonography without (A) and with compression (B) revealed deep vein thrombosis in the left calf vein. The blood flow signal means partial occlusion of the left calf veins. After compression, the deep vein thrombosis lesion showed no compressibility. However, deep vein thrombosis was initially undetected on the CT venograms.
C. On the retrospective analysis, a coronal CT image showed a small, non-occlusive thrombus (arrows) within the left calf vein.
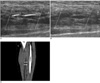
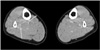
Fig. 4
A 32-year-old female underwent right total hip arthroplasty. An axial CT image showed non-enhancing, low-attenuated lesions surrounded by high-attenuated tissue in the right calf (arrows). However, there was no definite evidence of deep vein thrombosis in the right calf vein despite the repeated sonographic examinations. This was a false-positive case.
References
1. Elias A, Cadene A, Elias M, Puget J, Tricoire JL, Colin C, et al. Extended lower limb venous ultrasound for the diagnosis of proximal and distal vein thrombosis in asymptomatic patients after total hip replacement. Eur J Vasc Endovasc Surg. 2004. 27:438–444.
2. Nathan S, Aleem MA, Thiagarajan P, Das De S. The incidence of proximal deep vein thrombosis following total knee arthroplasty in an Asian population: a Doppler ultrasound study. J Orthop Surg (Hong Kong). 2003. 11:184–189.
3. Della Valle CJ, Steiger DJ, DiCesare PE. Duplex ultrasonography in patients suspected of postoperative pulmonary embolism following total joint arthroplasty. Am J Orthop. 2003. 32:386–388.
4. Sudo A, Sano T, Horikawa K, Yamakawa T, Shi D, Uchida A. The incidence of deep vein thrombosis after hip and knee arthroplasties in Japanese patients: a prospective study. J Orthop Surg (Hong Kong). 2003. 11:174–177.
5. Cordell-Smith JA, Williams SC, Harper WM, Gregg PJ. Lower limb arthroplasty complicated by deep venous thrombosis. Prevalence and subjective outcome. J Bone Joint Surg Br. 2004. 86:99–101.
6. Kim YH, Kim JS. Incidence and natural history of deep-vein thrombosis after total knee arthroplasty. J Bone Joint Surg Br. 2002. 84:566–570.
7. Kim YH, Oh SH, Kim JS. Incidence and natural history of deepvein thrombosis after total hip arthroplasty. A prospective and randomised clinical study. J Bone Joint Surg Br. 2003. 85:661–665.
8. Colwell CW Jr. Managing thromboembolic risk in hip and knee arthroplasty: state of the art. Orthopedics. 2003. 26:S231–S236.
9. Lieberman JR, Hsu WK. Prevention of venous thromboembolic disease after total hip and knee arthroplasty. J Bone Joint Surg Am. 2005. 87:2097–2112.
10. Mehta JS, Nicolaou N, Kiryluk S, Fordyce MJ. Venous leg ulcers after hip replacement. A clinical evaluation at 5 to 12 years. J Bone Joint Surg Br. 2003. 85:960–962.
11. Lee AY, Gent M, Julian JA, Bauer KA, Eriksson BI, Lassen MR, et al. Bilateral vs. ipsilateral venography as the primary efficacy outcome measure in thromboprophylaxis clinical trials: a systematic review. J Thromb Haemost. 2004. 2:1752–1759.
12. Wang CJ, Wang JW, Weng LH, Hsu CC, Lo CF. Outcome of calf deep-vein thrombosis after total knee arthroplasty. J Bone Joint Surg Br. 2003. 85:841–844.
13. Walker RH. Secondary prevention of venous thromboembolism in joint replacement using duplex ultrasonography. Orthopedics. 1994. 17:14–17.
14. Leutz DW, Stauffer ES. Color duplex Doppler ultrasound scanning for detection of deep venous thrombosis in total knee and hip arthroplasty patients. Incidence, location, and diagnostic accuracy compared with ascending venography. J Arthroplasty. 1994. 9:543–548.
15. Davidson BL, Elliott CG, Lensing AW. Low accuracy of color Doppler ultrasound in the detection of proximal leg vein thrombosis in asymptomatic high-risk patients. The RD Heparin Arthroplasty Group. Ann Intern Med. 1992. 117:735–738.
16. Elliott CG, Suchyta M, Rose SC, Talbot S, Ford C, Raskob G, et al. Duplex ultrasonography for the detection of deep vein thrombi after total hip or knee arthroplasty. Angiology. 1993. 44:26–33.
17. Garino JP, Lotke PA, Kitziger KJ, Steinberg ME. Deep venous thrombosis after total joint arthroplasty. The role of compression ultrasonography and the importance of the experience of the technician. J Bone Joint Surg Am. 1996. 78:1359–1365.
18. Ciccone WJ 2nd, Fox PS, Neumyer M, Rubens D, Parrish WM, Pellegrini VD Jr. Ultrasound surveillance for asymptomatic deep venous thrombosis after total joint replacement. J Bone Joint Surg Am. 1998. 80:1167–1174.
19. Eskandari MK, Sugimoto H, Richardson T, Webster MW, Makaroun MS. Is color-flow duplex a good diagnostic test for detection of isolated calf vein thrombosis in high-risk patients. Angiology. 2000. 51:705–710.
20. Robinson KS, Anderson DR, Gross M, Petrie D, Leighton R, Stanish W, et al. Ultrasonographic screening before hospital discharge for deep venous thrombosis after arthroplasty: the post-arthroplasty screening study. Ann Intern Med. 1997. 127:439–445.
21. Grady-Benson JC, Oishi CS, Hanson PB, Colwell CW Jr, Otis SM, Walker RH. Routine postoperative duplex ultrasonography screening and monitoring for the detection of deep vein thrombosis. A survey of 110 total hip arthroplasties. Clin Orthop Relat Res. 1994. 307:130–141.
22. Kalodiki E, Nicolaides AN, Al-Kutoubi A, Cunningham DA, Crofton M. Duplex scanning in the postoperative surveillance of patients undergoing total hip arthroplasty. J Arthroplasty. 1997. 12:310–316.
23. Robinson KS, Anderson DR, Gross M, Petrie D, Leighton R, Stanish W, et al. Accuracy of screening compression ultrasonography and clinical examination for the diagnosis of deep vein thrombosis after total hip or knee arthroplasty. Can J Surg. 1998. 41:368–373.
24. Westrich GH, Allen ML, Tarantino SJ, Ghelman B, Schneider R, Laskin RS, et al. Ultrasound screening for deep venous thrombosis after total knee arthroplasty: 2-year reassessment. Clin Orthop Relat Res. 1998. 356:125–133.
25. Verlato F, Bruchi O, Prandoni P, Camporese G, Maso G, Busonera F, et al. The value of ultrasnoud screening for proximal vein thrombosis after total hip arthroplasty. Thromb Haemost. 2001. 86:534–537.
26. Schwarcz TH, Matthews MR, Hartford JM, Quick RC, Kwolek CJ, Minion DJ, et al. Surveillance venous duplex is not clinically useful after total joint arthroplasty when effective deep venous thrombosis prophylaxis is used. Ann Vasc Surg. 2004. 18:193–198.
27. Westrich GH, Salvati EA, Sharrock N, Potter HG, Sanchez PM, Sculco TP. The effect of intraoperative heparin administered during total hip arthroplasty on the incidence of proximal deep vein thrombosis assessed by magnetic resonance venography. J Arthroplasty. 2005. 20:42–50.
28. Pookarnjanamorakot C, Sirisriro R, Eurvilaichit C, Jaovisidha S, Koysombatolan I. The incidence of deep vein thrombosis and pulmonary embolism after total knee arthroplasty: the screening study by radionuclide venography. J Med Assoc Thai. 2004. 87:869–876.
29. Szapiro D, Ghaye B, Willems V, Zhang L, Albert A, Dondelinger RF. Evaluation of CT time-density curves of lowerlimb veins. Invest Radiol. 2001. 36:164–169.
30. Garg K, Kemp JL, Wojcik D, Hoehn S, Johnston RJ, Macey LC, et al. Thromboembolic disease: comparison of combined CT pulmonary angiography and venography with bilateral leg sonography in 70 patients. AJR Am J Roentgenol. 2000. 175:997–1001.
31. Duwe KM, Shiau M, Budorick NE, Austin JH, Berkmen YM. Evaluation of the lower extremity veins in patients with suspected pulmonary embolism: a retrospective comparison of helical CT venography and sonography. AJR Am J Roentgenol. 2000. 175:1525–1531.
32. Begemann PGC, Bonacker M, Kemper J, Guthoff AE, Hahn KE, Steiner P, et al. Evaluation of the deep venous system in patients with suspected pulmonary embolism with multidetector CT: a prospective study in comparison to Doppler sonography. J Comput Assist Tomogr. 2003. 27:399–409.
33. Ghaye B, Szapiro D, Willems V, Dondelinger RF. Pitfalls in CT venography of lower limbs and abdominal veins. AJR Am J Roentgenol. 2002. 178:1465–1471.
34. Delis KT, Hunt N, Strachan RK, Nicolaides AN. Incidence, natural history and risk factors of deep vein thrombosis in elective knee arthroscopy. Thromb Haemost. 2001. 86:817–821.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download