Abstract
Percutaneous sacroplasty is a safe and effective procedure for sacral insufficient fractures under CT or fluoroscopic guidance; although, few reports exist about sacral metastatic tumors. We designed a pilot study to treat intractable pain caused by a sacral metastatic tumor with sacroplasty. A 62-year-old man and a 38-year-old woman with medically intractable pain due to metastatic tumors of S1 from lymphoma and lung cancer, respectively, underwent percutaneous sacroplasty. Over the course of the follow-up period, the two patients experienced substantial and immediate pain relief that persisted over a 3-month and beyond. The woman had deposition of PMMA (polymethyl methacrylate) in the needle track, but did not experience significant symptoms. No other peri-procedural complications were observed for either patient.
Sacroplasty is a safe and effective procedure in the treatment of sacral insufficient fractures under CT or fluoroscopic guidance (1, 2). Like vertebroplasty, it can also be used for skeletal metastatic tumors; however, few reports are described the literature (1, 3). Here, we report two cases with a description of the percutaneous sacroplasty method using bone cement injections for sacral metastatic tumors, under fluoroscopic guidance only.
A 62-year-old man affected by lymphoma with known skeletal metastases, was referred to our department with symptoms of refractory pain after non-operative therapy, which included chemotherapy, local radiotherapy, and opioid treatment. A month prior to the referral, the patient was admitted for a vertebroplasty for the T12, L1, L2 and L3 due to severe low back pain. The operation was significantly relieved from pain; however, the lower back pain progressively returned over the course of a month. Lumbosacral magnetic resonance imaging (MRI) and CT examinations (Fig. 1A, B) revealed an S1 mixed lesion. The patient had no weakness or neurosensory deficit.
After conferral with the orthopedic surgeon, discussion with the patient's family, and consent from the patient, percutaneous sacroplasty treatment was decided upon to restore sacrum stability and resolve the pain caused by the mixed lesion.
Prophylactic antibiotic therapy was performed by intravenous administration of 2 g of Cefuroxime Sodium (Livzon Medical Ltd, China), 30 minutes before commencing the procedure and was continued for 3 day after the procedure.
Before the procedure, a careful review of the radiology films and a measure of skin depth to the center of the lesion as well as the S1 marrow space from the dorsal to ventral cortex, were performed as specified by the trans-sacroiliac joint approach (Fig. 1C). The patient was placed in the prone position, comfortable and relaxed. Next, the lumbo-sacral region was prepped and draped to ensure sterility. The procedure was performed under local anesthesia. The sterile draped fluoroscope was positioned at the anteroposterior (AP) view. The lumbo-sacral junction was centered and the C-arm adjusted until the S1 foramen could be visualized. A small dermatotomy incision was made with a scalpel blade. Next, a 13-gauge bevel-edge needle (OptiMED Medizinische Instrumente GmbH, Germany) was introduced through the skin incision and subcutaneous tissue, to the periosteum of the bone, and then advanced to the center of the mixed lesion at the center of S1 transverse ilium, sacroiliac joint and sacrum (Fig. 1D, E) with the aid of a mallet. We adjusted the cephalopod and left-right angle according to the sagittal and axial films of CT and MRI until the needle attained the lesion under all fluoroscopy projections. After removal the inner needle, a commercial polymethyl methacrylate (PMMA) (Simplex P, Stryker Howmedica Osteonics, East Rutherford, NJ) was carefully injected carefully into the lesion under continuous fluoroscopic guidance in the lateral and AP projections (Fig. 1F, G) to ensure the adequate filling of the lesion and avoidance of PMMA leakage. A total of 6 ml of PMMA was injected. The post-procedural fluoroscopic findings showed optimal filling of the lesion with no evidence of PMMA extravasation.
The patient experienced significant pain relief the next day and was able to ambulate without assistance. The patient was discharged three days after the sacroplasty, following the completion of the prophylactic antibiotic therapy. Short-term (within 2 weeks) and middle-term (within 3 months) clinical follow-up were assessed by telephone. The physical examination elicited no pain upon palpation of the lumbosacral spine.
A 38-year-old woman was admitted to the hospital due to severe low back pain as a result of a sacral metastatic tumor associated with lung cancer (determined by a biopsy before the procedure). Both lumbo-sacral CT and MRI examinations showed an osteolytic change at the S1 level. The patient's image results revealed a destroyed cortex margin and was deemed not to be suitable for sacroplasty (Fig. 2A, B). However, the patient and her family strongly urged in favor of the operation, whatever the outcome. After obtaining informed consent, sacroplasty was performed under fluoroscopic guidance according to the technique described in Case 1. The procedure was well tolerated without significant complication except for the cement deposition in the needle track (Fig. 2C, D). Unfortunately, no CT images were available to display the distribution of PMMA to determine whether or not it leaked into neural foramina. However, the patient experienced considerable pain relief with no complaints of nerve root compression following the procedure. A 20 week post-operative follow-up over the telephone found that the patient experienced little pain and continued to walk independently.
Spinal metasatic tumors usually cause severe pain and immobility, requiring a high dose of opiates for pain relief (4). Currently, there is no ideal therapy available for the treatment of these lesions. Non-operative therapy for painful spinal metastasis includes bed rest, bracing, analgesia, radiotherapy, and chemotherapy, but the shortcoming of all these methods are that none can correct sacral instability. Surgical correction results in prolonged postoperative recovery time, with a high mortality rate 19% to 48% (5, 6), which is not a good option in patients with limited life expectancy.
Osteoplasty is an effective palliative treatment for symptomatic spinal metastases, relieving pain and improving quality of life (7, 8). The author's two sacroplasty cases provided marked pain relief, improved daily living activity, and no significant complications, except for cement disposition in the needle track in the female patient.
The mechanism of pain relief in patients with metastatic lesions treated by cement injection is currently not well understood. Stabilization of the micro-fractures and the analgesic effect of PMMA due to the thermal effect in itself seemed to be the main factors for short-term pain relief. Long-term pain relief will probably require volume reduction and necrosis of the tumor (9, 10).
Although the percutaneous sacroplasty technique is similar to vertebroplasty, there are several technical considerations to deal with which are not encountered when performing a vertebroplasty. Notably, it was difficult to locate the needle tip because the curved ventral cortical margin of the sacrum was difficult to see. Another concern was the risk of cement migration and the compression of the sacral nerve roots and sacral spinal canal.
Our two sacroplasties, performed entirely under fluoroscopic guidance, were successful; however, we recognize that the exclusive use of fluoroscopic guidance is problematic due to the difficulty in visualizing the sacral foramina. The visualization indicates whether the needle has adequately traversed the dorsal cortex and has not breached the ventral cortex, while also determining the distribution of PMMA. Although we did not encounter any complications related to the needle puncture and cement extravasation, this technique remains has potential risks.
The CT-guided approach gives better definition of the spatial relationships within the treatment field, which aids in preventing patient injury to the nearby vascular, neurological, and visceral structures. The CT also can provide more accurate imaging of complex fracture planes as well as enabling a higher level of precision in targeting the needle tip to the tumor. The relative limitation of CT-guidance is the lack of real-time monitoring of cement delivery and quick detection of stray cement extravasation.
A combination of the CT and fluoroscopic guidance may be the best alternative at present, which has been accepted by many authors (1, 3). Pommersheim et al. (2) suggest a CT-fluoroscopy suite might be the ideal setting to allow for both precise needle placement and real-time visualization of cement delivery.
In conclusion, sacroplasties may be performed under fluoroscopic guidance only, although they are technically demanding and involve an increased risk of injury. Our success in performing the sacroplasties was attributed to performing 837 vertebroplasties over a six year period. We recommend that physicians have extensive experience in performing vertebroplasties before attempting sacral repair.
Figures and Tables
Fig. 1
62-year-old man with metastasis at T12, L1, L2, L3, and S1 level from lymphoma.
A, B. Sagittal CT (A) shows mixed sclerotic and poorly marinated low attenuation lesion in S1. Sagittal T2-weighted with fat saturated MR image (B) shows high band-like signal at periphery and iso-to-low signal in central portion of lesion.
C. Transaxial schematic drawing demonstrates that trans-sacroiliac joint approach and positioning of 13-gauge bevel-edge needle is recommended for sacroplasties. Needle courses ilium, sacroiliac joint and sacral pedicle to center of lesion. Rotating needle to aim bevel-edge needle toward nerve root is recommended to avoid injury.
D, E. Lateral (D) and anteroposterior (E) fluoroscopic versions reveal location of needle tip in lesion.
F, G. Lateral (F) and anteroposterior (G) post-operative fluoroscopic views show equal distribution of cement with no cement migration and leakage at T12, L1, L2, L3, and S1.
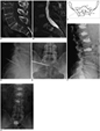
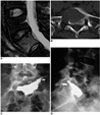
Fig. 2
38-year-old woman with metastatic tumor at S1, originating from lung cancer.
A, B. Sagittal T2-weighted with fat saturated MR imaging (A) shows abnormal signal at S1. Axial CT scan (B) demonstrates osteolytic change of S1, which is characterized by breakdown of peri-cortex of S1 and S1 foramen.
C, D. Anteroposterior (C) and lateral (D) views of postoperative fluoroscopy shows no cement migration. In addition, there is little cement leakage from needle track (arrows). Patient had no significant symptoms.
References
1. Strub WM, Hoffmann M, Ernst RJ, Bulas RV. Sacroplasty by CT and fluoroscopic guidance: is the procedure right for your patient? AJNR Am J Neuroradiol. 2007. 28:38–41.
2. Pommersheim W, Huang-Hellinger F, Baker M, Morris P. Sacroplasty: a treatment for sacral insufficient fractures. AJNR Am J Neuroradiol. 2003. 24:1003–1007.
3. Dehdashti AR, Martin JB, Jean B, Rufenacht DA. PMMA cementoplasty in symptomatic metastatic lesions of the S1 vertebral body. Cardiovasc Intervent Radiol. 2000. 23:235–237.
4. Uemura A, Fujimoto H, Yasuda S, Osaka I, Goto N, Shinozaki M, et al. Transcatheter arterial embolization for bone metastases from hepatocelluar carcinoma. Eur Radiol. 2001. 11:1457–1462.
5. Pascal-Moussellard H, Broc G, Pointillart V, Simeon F, Vital JM, Senegas J. Complications of vertebral metastasis surgery. Eur Spine J. 1998. 7:438–444.
6. Sundaresan N, Sachdev VP, Holland JF, Moore F, Sung M, Paciucci PA, et al. Surgical treatment of spinal cord compression from epidural metastasis. J Clin Oncol. 1995. 13:2330–2335.
7. Kelekis A, Lovblad KO, Mehdizade A, Somon T, Yilmaz H, Wetzel SG, et al. Pelvic osteoplasty in osteolytic metastases: technical approach under fluoroscopic guidance and early clinical results. J Vasc Interv Radiol. 2005. 16:81–88.
8. Li AF, Li KC, Chang FY, Hsieh CH. Preliminary report of transpedicle body augmenter vertebroplasty in painful vertebral tumors. Spine. 2006. 31:E805–E812.
9. Baroud G, Samara M, Steffen T. Influence of mixing method on the cement temperature-mixing time history and doughing time of three acrylic cements for vertebroplasty. J Biomed Mater Res B Appl Biomater. 2004. 68:112–116.
10. Belkoff SM, Molloy S. Temperature measurement during polymerization of polymethylmethacrylate cement used for vertebroplasty. Spine. 2003. 28:1555–1559.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download