Abstract
Materials and Methods
Fetal gall bladder development was evaluated in well-dated, non-anomalous fetuses in the Korean population between February and April 2003 and the visualization rate and reference values were determined from the obtained data.
Results
The visualization rate of the fetal gall bladder increased as gestation advanced to a plateau above 90%, which was maintained between 16 and 34 weeks. The measured parameters from the fetal gall bladder had a significant positive relationship with gestational age (p = 0.000 for all cases), and the correlation of length and area with the gestational age (r = 0.741 and r = 0.690, respectively) was better than the correlation of width, height, and volume with gestational age. The repeatability coefficients and coefficients of variation between the two operators were 5.56 mm and 12.9% for the length and 344.11 mm2 and 33.52% for the area. The median length of the fetal gall bladder in the Korean population was not significantly different from the mean length of gall bladders in the Caucasian and African-American populations (p = 0.915).
The fetal gall bladder, which is commonly seen during antenatal sonography, usually presents as an anechoic, elliptical structure laterally right of the intrahepatic umbilical vein. The gall bladder grows in a predictable fashion with regards to advance in gestation (1, 2), however, an unusually large gall bladder is often seen. Because a large gall bladder is a well-known postnatal feature of trisomy 13 (3), size evaluation of the fetal gall bladder may indicate if there is a risk of chromosomal aneuploidy. For this reason, several studies have provided reference values for the fetal gall bladder throughout gestation, however, the data generated by these studies has been limited to the Caucasian and African-American populations (1, 2). To our knowledge, no data has been published regarding reference values of the fetal gall bladder in Asians; therefore, this study was conducted with the intent of providing reference ranges for the fetal gall bladder throughout gestation in the Korean population.
We prospectively evaluated the fetal gall bladder in low-risk pregnant women who visited our unit for routine antenatal sonography between February and April 2003. Gestational age (GA) was calculated according to the first date of the last menstrual period, and confirmed by the first-trimester sonographic measurements. The study population consisted of pregnancies which fulfilled the following criteria: 1) history of regular menses with a known date of the beginning of the last menstrual period, 2) a discrepancy of less than seven days between the GA predicted by the last menstrual period and that estimated by first-trimester sonography, 3) singleton pregnancy, 4) absence of maternal disease known to affect normal fetal growth, 5) a clinically-normal fetus at birth. To maintain a properly designed cross-sectional study, each fetus participated only once during gestation. Our institutional review board approved the study, and verbal informed consent was obtained from each woman evaluated.
Ultrasounds were conducted using a Voluson 730,LOGIQ 400, 500, or 5 (GE Medical Systems, Milwaukee,WI), or an Ultramark 9 system (Philips Medical Systems, Bothell, WA). The ultrasound evaluations were performed by seven experienced sonographers certified by the American Registry of Diagnostic Medical Sonographers, two of which had four years experience and one each with 5, 6, 7, 9 and 11 years experience. Evaluation of the fetal gall bladder was conducted as a standard part of prenatal sonographic examinations. Identification of the fetal gall bladder was done in the axial plane just below the level of the stomach. Color Doppler imaging was employed as necessary to confirm that the gall bladder had not been confused with the intraabdominal umbilical vein. If the fetal gall bladder was not found during the initial search, the fetal abdomen was intermittently rescanned in an attempt to observe the fetal gall bladder. If the fetal gall bladder was identified, the transducer was tilted to visualize the gall bladder in its maximal length, and length and width of the fetal gall bladder were measured using electronic calipers at an increment of 0.1 mm. The height of the fetal gall bladder was measured along the transverse section of the gall bladder, which was acquired after the transducer was rotated 90 degrees perpendicular to the long axis of the fetal gall bladder (Fig. 1). To evaluate the reliability of US, the three dimensional diameters, width and volume of the gall bladder was measured in the fetuses of 20 randomly-sampled pregnant women between 20 and 36 weeks of gestation by two observers with 11 years and four years of experience, respectively. Each observer performed each measurement twice and the mean value was taken as the representative values of each observer. Area and volume were calculated using the following formulas; area = 3.14 * ([width + length]/2) 2, volume = 0.523 * width * height * length. When three measurements of the same fetal gall bladder were available, the obtained data were used to establish reference values based on the gestational age.
Statistical analyses were performed using commercially available software packages (SPSS version 10.0, SPSS, Chicago, IL). Scatter plots of the analyzed parameters (length, width, height, area, and volume) as a function of gestational age were generated and their relationships were assessed using the Pearson correlation analysis. Pearson correlation coefficients were calculated to evaluate the strength of the relationship between measured parameters and gestational age. Reference values were acquired by stratifying the fetuses into eight subgroups on the basis of their gestational age, and then calculating reference values (median, 5th percentile, 95th percentile) for each subgroup. The gall bladder lengths in our study were compared with those of Caucasian and African-American populations using the Wilcoxon signed rank sum test. In the latter, the median values were 1.0 cm at 15-19 weeks, 1.5 cm at 20-22 weeks, 1.9 cm at 23-24 weeks, 2.1 cm at 25-26 weeks, 2.1 cm at 27-30 weeks, 2.6 cm at 31-34 weeks, and 2.7 cm at 35-40 weeks (2). A p 0.05 was considered to indicate a significant difference. The reliability of our measurements was evaluated using the Bland and Altman plot, with the repeatability coefficient being used to demonstrate interobserver agreement (4). The coefficient of variation was calculated to express the ratio of the standard deviation (SD) to the mean value.
Sonographic visualization and measurement of the fetal gall bladder was possible at as early as 12 weeks gestation. Our study population consisted of 1,911 of the 2,170 fetuses enrolled in this study (88.1%) that produced results meeting the acceptable criteria. Of these, the gall bladders of 1,417 fetuses (74.1%) were visualized using antenatal sonography. Visualization of the gall bladder was achieved in greater than 90% of women at gestation periods between 16 and 34 weeks, however the gall bladder was identified in less than 90% of study population after 35 weeks (Fig. 2). Measurements of the length, width, and height of the fetal gall bladder were obtained from 1,292 of 1,417 fetuses (91.2%), however the scanning plane could not be adjusted to depict the maximal length, width, or height of the gall bladder in the remaining 125 fetuses. Table 1 shows fetal gall bladder length, height, width, area and volume according to gestational age. The measured parameters of the fetal gall bladder had a significant positive relationship with gestational age (Table 2). The correlation of length and area to gestational age was better than that of width, height, and volume, therefore, reference values based on the gestational age were only calculated for the length and area (Table 3). The median values of the gall bladder lengths in the Korean population were not significantly different from those in the Caucasian and African-American populations (p = 0.915).
The mean differences, repeatability coefficients, and coefficients of variation between the two operators were 1.85 ± 2.84 mm (mean ± SD), 5.56 mm, and 12.9% for the length, 0.56 ± 1.14 mm, 2.23 mm, and 21.0% for the width, 0.25 ± 0.83 mm, 1.63 mm, and 16.6% for the height, 125.66 ± 175.56 mm2, 344.11 mm2, and 33.52% for the area, and 110.07 ± 217.54 mm3, 426.37 mm3, 97.36% for the volume.
It has been demonstrated that growth of fetal organs varies between ethnicities, even when environmental and socio-economic factors are considered (5-8). The fetal gall bladder can commonly be seen as a fluid filled structure during antenatal sonography and the normative data of the fetal gall bladder throughout gestation has been established in Caucasian and African-American populations (1-2). The application of those normative data may be inappropriate for evaluation of the fetal gall bladder in other populations, however, if ethnic differences between populations have not been compared. In our larger study of 1,911 fetuses, we provided reference values for the growth of the fetal gall bladder throughout gestation in the Korean population. The lengths of the fetal gall bladder were not statistically different from those of the Caucasian and African-American populations, which suggests that there is no need to consider ethnicity when interpreting the size of fetal gall bladder in Asian, African-American and Caucasian populations.
Some investigators have asserted that the fetal gall bladder can always be observed after the early part of the second trimester, therefore non-visualization of the fetal gall bladder after this point may indicate cystic fibrosis, gall bladder atresia, and biliary atresia (9-10). Hertzberg et al. have refuted this assertion, however, in a study that assessed the prognostic importance of non-visualization of the gall bladder during gestation (11). In their study, the fetal gall bladder was not seen in approximately 5% of fetuses at 24-32 weeks gestation, however a normal outcome occurred in most of the cases in which the gall bladder of the fetus could not be visualized. In our study, the gall bladder was not seen in 84 (6.8%) fetuses at 16-34 weeks gestation, which is similar to the findings of Hetzberg et al. Moreover, the visualization rate of the fetal gall bladder decreased in fetuses scanned after 35 weeks of gestation, which might be attributable to either technical difficulty or increased gall bladder contractility with advanced gestation (11, 12). Therefore, our findings support Hertzberg's opinion (11) that the rate of non-visualization of the fetal gall bladder is sufficiently high, indicating that non-visualization of the gall bladder may not be a useful screen for fetal anomalies such as biliary atresia.
It should be noted that our study was limited because it lacked a true reference standard for validating normal neonatal outcomes in the study population. While we excluded all fetuses with abnormalities that were believed to have a potential influence on the normality of the fetal gall bladder, we cannot be completely certain that this exclusion was effective. However, we believe that such a limitation is unavoidable when conducting prenatal imaging research to provide reference values for the general population. Another limitation of this study is that the analysis of interobserver variability used a relatively low number of women and intraobserver variability was not analyzed. Although each observer performed the measurement twice, it was performed without appropriate time intervals between two measurements, and thus intraobserver variability could not be analyzed.
In summary, we have provided reference values of the fetal gall bladder throughout gestation in the Korean population. As shown in our study, the reference values of the fetal gall bladder in the Korean population are not statistically different from those of the Caucasian and African-American populations, and non-visualization of the fetal gall bladder is a relatively common finding with little clinical significance as a sign of fetal abnormality.
Figures and Tables
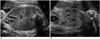
Fig. 1
Sonogram demonstrating fetal gall bladder length and width (A) and height (B). The height was obtained by rotating the transducer 90 degree from plane (A) showing maximal length and width of the gall bladder. The caliper placement for measurement of the gall bladder is demonstrated. S = spine, B = bladder
References
1. Chan L, Rao BK, Jiang Y, Endicott B, Wapner RJ, Reece EA. Fetal gallbladder growth and development during gestation. J Ultrasound Med. 1995. 14:421–425.
2. Goldstein I, Tamir A, Weisman A, Jakobi P, Copel JA. Growth of the fetal gall bladder in normal pregnancies. Ultrasound Obstet Gynecol. 1994. 4:289–293.
3. Jones KL. Jones KL, editor. Chromosomal abnormality syndrome. Smith's recognizable patterns of human malformations. 1988. Philadelphia: Saunders;10–25.
4. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999. 8:135–160.
5. Bromley B, Frigoletto FD Jr, Harlow BL, Evans JK, Benacerraf BR. Biometric measurements in fetuses of different race and gender. Ultrasound Obstet Gynecol. 1993. 3:395–402.
6. Davis RO, Cutter GR, Goldenberg RL, Hoffman HJ, Cliver SP, Brumfield CG. Fetal biparietal diameter, head circumference, abdominal circumference and femur length. A comparison by race and sex. J Reprod Med. 1993. 38:201–206.
7. Jacquemyn Y, Sys SU, Verdonk P. Fetal transverse cerebellar diameter in different ethnic groups. J Perinat Med. 2000. 28:14–19.
8. Moon MH, Cho JY, Lee YM, Lee YH, Yang JH, Kim MY, et al. Nasal bone length at 11-14 weeks of pregnancy in the Korean population. Prenat Diagn. 2006. 26:524–527.
9. Bronshtein M, Weiner Z, Abramovici H, Filmar S, Erlik Y, Blumenfeld Z. Prenatal diagnosis of gall bladder anomalies-report of 17 cases. Prenat Diagn. 1993. 13:851–861.
10. Duchatel F, Muller F, Oury JF, Mennesson B, Boue J, Boue A. Prenatal diagnosis of cystic fibrosis: ultrasonography of the gallbladder at 17-19 weeks of gestation. Fetal Diagn Ther. 1993. 8:28–36.
11. Hertzberg BS, Kliewer MA, Maynor C, McNally PJ, Bowie JD, Kay HH, et al. Nonvisualization of the fetal gallbladder: frequency and prognostic importance. Radiology. 1996. 199:679–682.
12. Tanaka Y, Senoh D, Hata T. Is there a human fetal gallbladder contractility during pregnancy? Hum Reprod. 2000. 15:1400–1402.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download